|
1
|
Fe
|

|
Iron
|
7.874
|
26
|
56
|
1811
|
3134
|
449
|
1.83
|
1043
|
2.22
|
1.75
|
Body-centered cubic (bcc)
|
Lustrous metallic
|
Transition metal
|
Ferromagnetic
|
Before 5000 BC
|
Iron is a chemical element with symbol Fe (from Latin: ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is by mass the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust.
|
https://cdn.pixabay.com/photo/2014/05/16/16/06/pyrite-345637_960_720.jpg
|
https://en.wikipedia.org/wiki/Iron
|
View
|
|
2
|
Co
|

|
Cobalt
|
8.9
|
27
|
59
|
1768.15
|
3200
|
421
|
1.88
|
1400
|
1.72
|
1.45
|
Hexagonal close-packed (hcp)
|
Bluish-white
|
Transition metal
|
Ferromagnetic
|
Georg Brandt (1735)
|
Cobalt is a bluish-white, lustrous, hard, brittle metal. It is ferromagnetic. The metal is active chemically, forming many compounds. Cobalt stays magnetic to the highest temperature of all the magnetic elements (it has a Curie point of 1121oC).
|
https://www.sciencefriday.com/wp-content/uploads/2019/03/yinmnblue_nocredit.png
|
https://www.chemicool.com/elements/cobalt.html
|
View
|
|
3
|
Ni
|

|
Nickel
|
8.91
|
28
|
58.6
|
1726.15
|
3186
|
445
|
1.91
|
631
|
0.62
|
0.5
|
Face-centered cubic (fcc)
|
Silvery-white lustrous metal
|
Transition metal
|
Ferromagnetic
|
Axel Fredrik Cronstedt (1751)
|
Nickel is a naturally-occurring metallic element with a silvery-white, shiny appearance. It is the fifth-most common element on earth and occurs extensively in the earth’s crust and core. Nickel, along with iron, is also a common element in meteorites.
|
https://www.collinsdictionary.com/images/thumb/nickel_341165792_250.jpg
|
https://nickelinstitute.org/about-nickel/
|
View
|
|
4
|
Gd
|

|
Gadolinium
|
7.9
|
64
|
157.25
|
1585
|
3523
|
240
|
1.2
|
292
|
7.63
|
2
|
Hexagonal close-packed (hcp)
|
Silvery white
|
Lanthanide
|
Ferromagnetic
|
Jean Charles Galissard de Marignac (1880)
|
Gadolinium is a chemical element that is on the Periodic Table of the Elements with the atomic number of 64. It is a silvery-white metal that reacts with bodily molecules during an MRI scan.
|
https://www.soundimaginginc.com/images/gadolinium_.jpg
|
https://www.independentimaging.com/what-is-gadolinium/
|
View
|
|
5
|
Cr
|

|
Chromium
|
7.19
|
24
|
52
|
2180
|
2944
|
448
|
1.66
|
|
|
|
Body-centered cubic (bcc)
|
Silvery metallic
|
Transition metal
|
Antiferromagnetic
|
Louis Nicolas Vauquelin
|
Chromium is a blue-white metal that is hard, brittle and very corrosion resistant. Chromium can be polished to form a very shiny surface and is often plated to other metals to form a protective and attractive covering.
|
https://upload.wikimedia.org/wikipedia/commons/a/a1/Chromium.jpg
|
https://education.jlab.org/itselemental/ele024.html
|
View
|
|
6
|
Sm
|

|
Samarium
|
7.353
|
62
|
150.36
|
1345
|
2076
|
196
|
1.17
|
|
|
|
Simple Trigonal
|
Silver
|
Lanthanide
|
Paramagnetic
|
Lecoq de Boisbaudran (1879)
|
Samarium is a rare earth metal, with a bright silver luster, that is reasonably stable in air; it ignites in air at 150 °C. Even with long-term storage under mineral oil, samarium is gradually oxidized, with a grayish-yellow powder.
|
https://periodictable.com/Samples/062.6/s9s.JPG
|
https://www.chemeurope.com/en/encyclopedia/Samarium.html
|
View
|
|
7
|
Pt
|
|
Platinum
|
21.45
|
78
|
195
|
2041.4
|
4098
|
133
|
2.28
|
|
|
|
Face-centered cubic (fcc)
|
Silvery white
|
Transition metal
|
Paramagnetic
|
Antonio de Ulloa (1735)
|
Platinum buried deep in the middle of the periodic table among elements collectively known as the transition metals. Its near neighbors include iridium, osmium, palladium, rhodium, and ruthenium which, with platinum, called the platinum group metals.
|
https://cdn.pixabay.com/photo/2016/04/23/11/14/cube-1347427_960_720.png
|
https://www.explainthatstuff.com/platinum.html
|
View
|
|
8
|
Al
|

|
Aluminium
|
2.7
|
13
|
27
|
660.32
|
2519
|
904
|
1.61
|
|
|
|
Face-centered cubic (fcc)
|
Silvery gray metallic
|
Post-transition metal
|
Paramagnetic
|
Hans Christian Ørsted (1824)
|
Aluminum (Al) is the 13th element on the periodic table, and is the most abundant metal on Earth, making up 8.1% of the Earth's crust. It is not found freely in nature, but it is always found combined to other elements due to it being very reactive.
|
https://www.azom.com/images/Article_Images/ImageForArticle_1446(1).jpg
|
https://energyeducation.ca/encyclopedia/Aluminum
|
View
|
|
9
|
Ba
|

|
Barium
|
3.51
|
56
|
137
|
1000
|
2143
|
205
|
0.89
|
|
|
|
Body-centered cubic (bcc)
|
Silvery gray
|
Alkaline earth metal
|
Paramagnetic
|
Carl Wilhelm Scheele (1772)
|
Barium is an active metal. It combines easily with oxygen, the halogens, and other non-metals. The halogens are Group 17 of the periodic table and include fluorine, chlorine, bromine, iodine, and astatine. Barium also reacts with water and most acids.
|
https://clearlyexplained.com/_Media/barium_med_hr.jpeg
|
http://www.chemistryexplained.com/elements/A-C/Barium.html
|
View
|
|
10
|
Sr
|

|
Strontium
|
2.63
|
38
|
87.62
|
1050
|
1655
|
300
|
0.95
|
|
|
|
Face-centered cubic (fcc)
|
Silvery white metallic
|
Alkaline earth metal
|
Paramagnetic
|
William Cruickshank (1790)
|
Strontium is a silvery-yellow, metallic element. Strontium belongs to a group of elements known as the alkali earth metals. Like other alkali metals, it is chemically active and will react with both air and water.
|
https://www.collinsdictionary.com/images/full/strontium_389727430.jpg
|
https://mineralseducationcoalition.org/minerals-database/strontium/
|
View
|
|
11
|
Dy
|

|
Dysprosium
|
8.54
|
66
|
162.5
|
1680
|
2840
|
167
|
1.22
|
87
|
10.2
|
|
Hexagonal close-packed (hcp)
|
Silvery white
|
Lanthanide
|
Paramagnetic
|
Lecoq de Boisbaudran (1886)
|
Dysprosium metal is a soft, lustrous-silver rare earth element (REE) that is used in permanent magnets due to its paramagnetic strength and high-temperature durability.
|
https://www.thoughtco.com/thmb/rER8hJycbJY7V1MZsmExLSoPTlc=/1436x1138/filters:fill(auto,1)/Dy-Metal-2-56a613dd5f9b58b7d0dfcc4a.jpg
|
https://www.thoughtco.com/metal-profile-dysprosium-2340187
|
View
|
|
12
|
Pd
|

|
Palladium
|
12.02
|
46
|
106.42
|
1828.05
|
3236
|
240
|
2.2
|
|
|
|
Face-centered cubic (fcc)
|
Silvery white
|
Transition metal
|
Paramagnetic
|
William Hyde Wollaston (1803)
|
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas.
|
https://upload.wikimedia.org/wikipedia/commons/thumb/2/23/Palladium.jpg/260px-Palladium.jpg
|
https://www.britannica.com/science/palladium-chemical-element
|
View
|
|
13
|
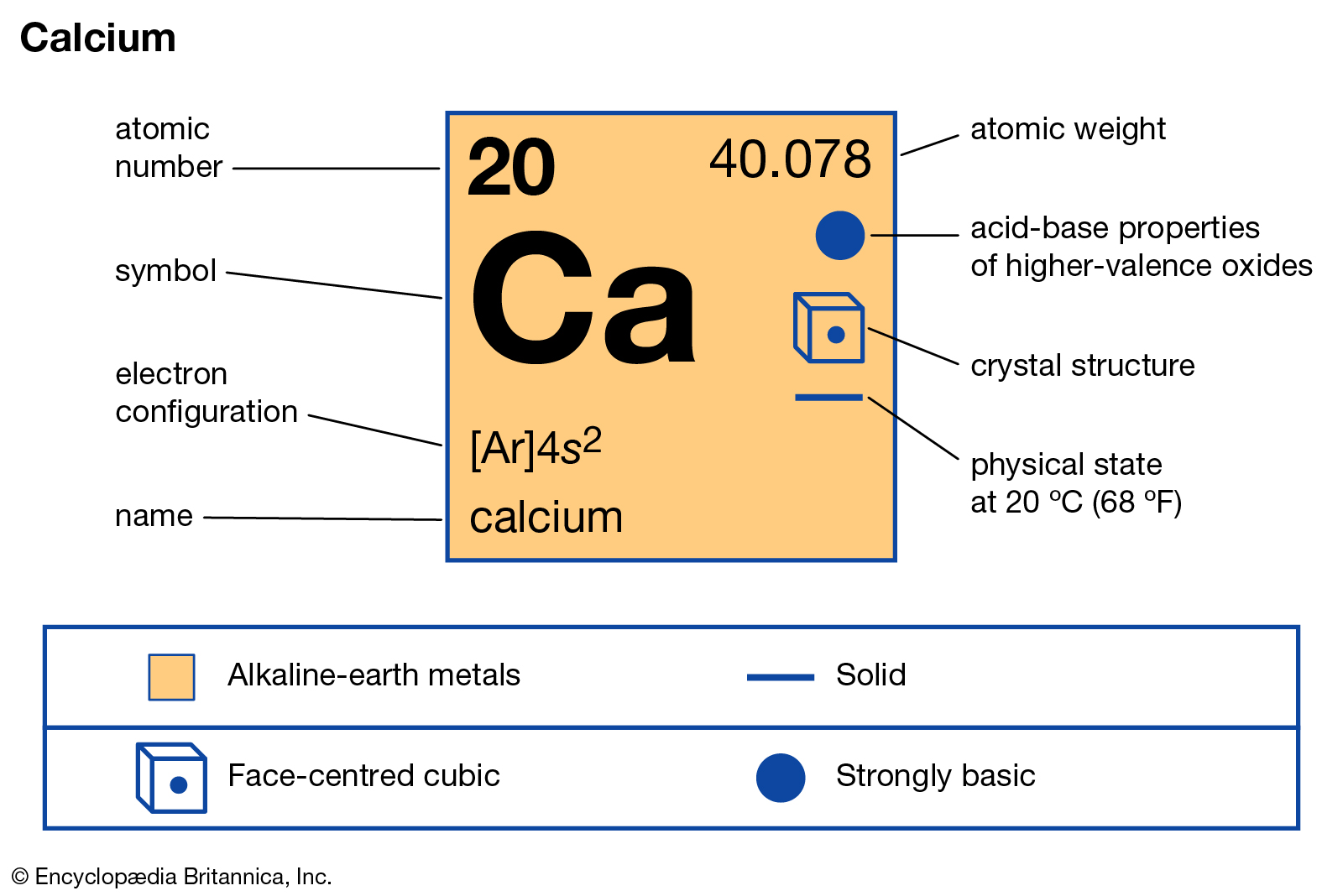
Ca
|
|
Calcium
|
1.55
|
20
|
40
|
1115
|
1757
|
631
|
1
|
|
|
|
Face-centred cubic (fcc)
|
Dull gray, silver
|
Alkaline earth metal
|
Paramagnetic
|
In The United Kingdom (1808)
|
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium.
|
https://cdn.britannica.com/80/22380-050-56F1E872/Calcium-earth-metal-symbol-square-Ca-properties.jpg
|
https://www.rsc.org/periodic-table/element/20/calcium
|
View
|
|
14
|
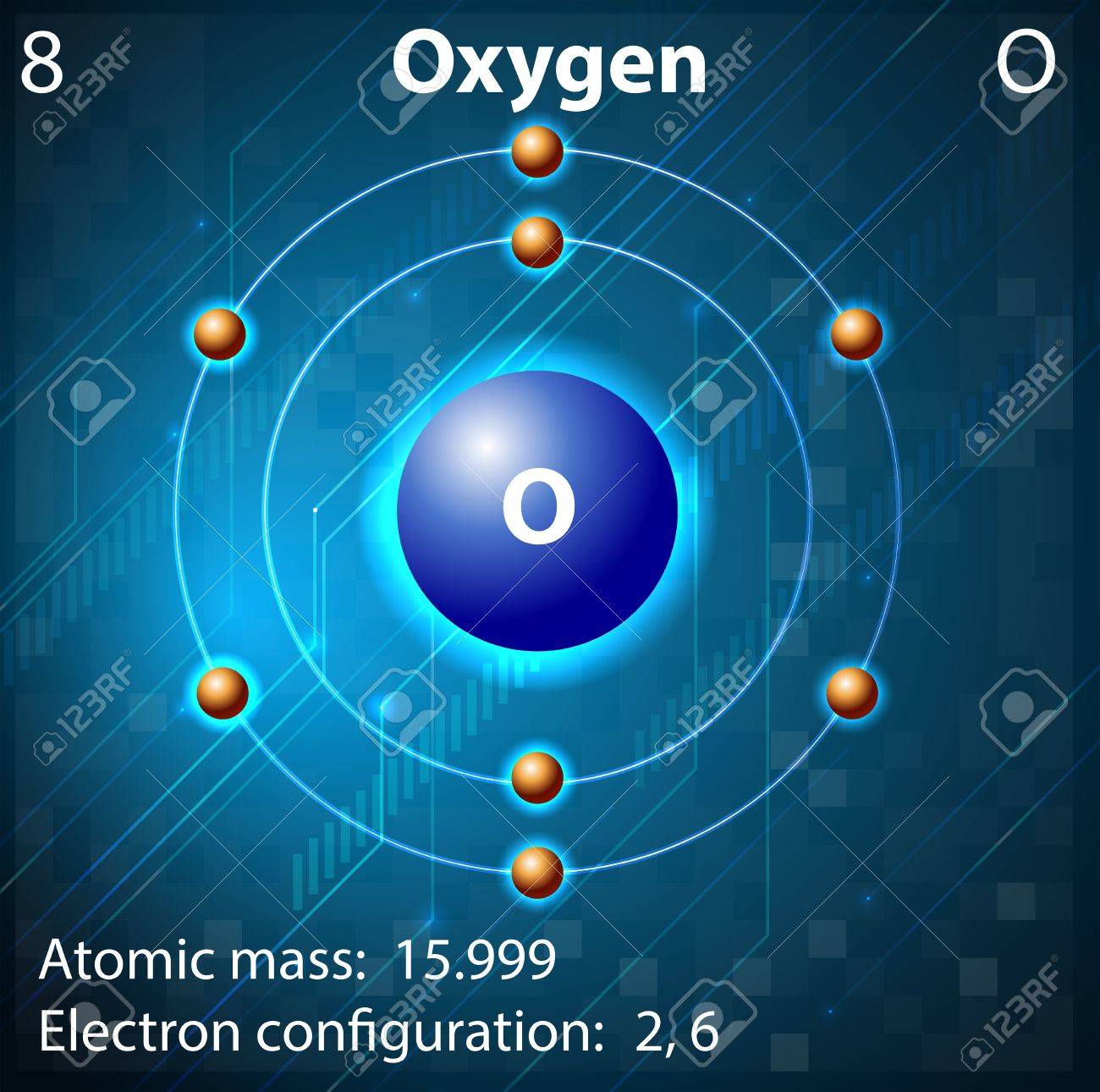
O
|
|
Oxygen
|
1.429
|
8
|
16
|
54.8
|
90.2
|
919
|
3.44
|
|
|
|
Base-centered Monoclinic
|
Gas, colorless
|
Reactive nonmetal
|
Paramagnetic
|
Carl Wilhelm Scheele (1774)
|
Oxygen is the third most abundant element in the universe and makes up nearly 21% of the earth's atmosphere. Oxygen accounts for nearly half of the mass of the earth's crust, two thirds of the mass of the human body and nine tenths of the mass of water. Large amounts of oxygen can be extracted from liquefied air through a process known as fractional distillation. Oxygen can also be produced through the electrolysis of water or by heating potassium chlorate (KClO3).
|
https://previews.123rf.com/images/blueringmedia/blueringmedia1309/blueringmedia130900040/40669048-illustration-of-the-element-oxygen.jpg
|
https://education.jlab.org/itselemental/ele008.html
|
View
|
|
15
|
Mn
|

|
Manganese
|
7.47
|
25
|
55
|
1519
|
2334
|
479
|
1.55
|
|
|
|
Body-centered cubic (bcc)
|
Silvery metallic
|
Transition metal
|
Paramagnetic
|
Carl Wilhelm Scheele (1774)
|
Manganese is used in ceramics, glass, dyes, dry cell batteries, and special high carbon steels. It is also added to fertilizers and animal food.
|
https://upload.wikimedia.org/wikipedia/commons/8/86/Mangan_1-crop.jpg
|
https://doi.org/10.1016/B978-0-12-386454-3.00873-3
|
View
|
|
16
|
Nd
|

|
Neodymium
|
7.01
|
60
|
144.24
|
1294
|
3373
|
190
|
1.14
|
|
|
|
Double hexagonal close-packed (dhcp)
|
Malleable silvery metal
|
Lanthanide
|
Paramagnetic
|
Carl Auer von Welsbach (1885)
|
Neodimium is a lustrous silvery-yellow metal. It is very reactive and qickly turnishes in air and the coated formed does not protect the metal from further oxidation, so it must be stored away from contact with air.
|
https://images-of-elements.com/neodymium-ox.jpg
|
https://www.lenntech.com/periodic/elements/nd.htm
|
View
|
|
17
|
B
|

|
Boron
|
2.46
|
5
|
10.81
|
2348
|
4273
|
1030
|
2.04
|
|
|
|
Rhombohedral
|
Black-brown
|
Metalloid
|
Diamagnetic
|
Joseph Louis Gay-Lussac and Louis Jacques Thénard (30 June 1808)
|
Boron is a chemical element with the symbol B and atomic number 5. Produced entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, it is a low-abundance element in the Solar System and in the Earth's crust.
|
https://en.wikipedia.org/wiki/Boron
|
https://en.wikipedia.org/wiki/Boron
|
View
|
|
18
|
Ge
|

|
Germanium
|
5.323
|
32
|
72.63
|
1211.4
|
3093
|
321
|
2.01
|
|
|
|
Face-centered diamond-cubic
|
Grayish-white
|
Metalloid
|
Diamagnetic
|
Clemens Winkler (1886)
|
Germanium (Ge), a chemical element between silicon and tin in Group 14 (IVa) of the periodic table, a silvery-gray metalloid, intermediate in properties between the metals and the nonmetals.
|
https://d2cbg94ubxgsnp.cloudfront.net/Pictures/480xany/3/6/1/143361_shutterstock_703383247.jpg
|
https://www.britannica.com/science/germanium
|
View
|
|
19
|
Cu
|

|
Copper
|
8.96
|
29
|
63
|
1357.77
|
2835
|
385
|
1.9
|
|
|
|
Face-centered cubic (fcc)
|
Red-orange metallic luster
|
Transition metal
|
Diamagnetic
|
Middle East (9000 BC)
|
Copper ranks as the third-most-consumed industrial metal in the world, after iron and aluminum, according to the U.S. Geological Survey (USGS). About three-quarters of that copper goes to make electrical wires, telecommunication cables and electronics.
|
https://cdn.mos.cms.futurecdn.net/shmcZe8p34XX39QFAGiJHG.jpg
|
https://www.livescience.com/29377-copper.html
|
View
|
|
20
|
Si
|

|
Silicon
|
2.329
|
14
|
28
|
1687
|
3173
|
710
|
1.9
|
|
|
|
Tetrahedral Packing
|
Gray
|
Metalloid
|
Diamagnetic
|
Jöns Jacob Berzelius (1824)
|
Silicon is the seventh-most abundant element in the universe and the second-most abundant element on the planet, after oxygen, according to the Royal Society of Chemistry. About 25 percent of the Earth's crust is silicon.
|
https://www.livescience.com/28893-silicon.html
|
https://www.livescience.com/28893-silicon.html
|
View
|
|
21
|
C
|

|
Carbon
|
2.26
|
6
|
12
|
3823
|
4300
|
0
|
2.55
|
|
|
|
Simple hexagonal
|
Graphite black
|
Reactive nonmetal
|
Diamagnetic
|
Egyptians and Sumerians (3750 BCE)
|
Carbon, the sixth most abundant element in the universe, has been known since ancient times. Carbon is most commonly obtained from coal deposits, although it usually must be processed into a form suitable for commercial use.
|
https://media.sciencephoto.com/c0/47/53/48/c0475348-800px-wm.jpg
|
https://education.jlab.org/itselemental/ele006.html
|
View
|
|
22
|
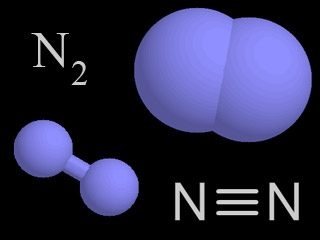
N
|
|
Nitrogen
|
1.251
|
7
|
14
|
63.05
|
77.36
|
1040
|
3.04
|
|
|
|
Simple Hexagonal
|
Colorless gas, liquid or solid
|
Reactive nonmetal
|
Diamagnetic
|
Daniel Rutherford (1772)
|
Nitrogen is a chemical element with an atomic number of 7 (it has seven protons in its nucleus). Molecular nitrogen (N2) is a very common chemical compound in which two nitrogen atoms are tightly bound together.
|
https://scied.ucar.edu/nitrogen
|
https://scied.ucar.edu/nitrogen
|
View
|
|
23
|
Ne
|

|
Neon
|
0.9
|
10
|
20.1
|
24.56
|
27.07
|
1030
|
|
|
|
|
Face-centered cubic (fcc)
|
Colorless gas exhibiting an orange-red glow when placed in an electric field
|
Noble gas
|
Diamagnetic
|
William Ramsay & Morris Travers (1898)
|
The chemical element neon is classed as a noble gas and a nonmetal. It was discovered in 1898 by William Ramsay and Morris Travers. Ramsay was aware an element must sit between helium and argon in the periodic table.
|
https://cdn.britannica.com/90/22390-050-6623F39C/Neon-neon-symbol-square-properties-some-periodic.jpg
|
https://www.chemicool.com/elements/neon.html
|
View
|
|
24
|
Zn
|

|
Zinc
|
7.14
|
30
|
65.38
|
692.68
|
1180
|
388
|
1.65
|
|
|
|
Hexagonal close-packed (hcp)
|
Silver-gray
|
Transition metal
|
Diamagnetic
|
Indian metallurgists (before 1000 BCE)
|
Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Metallic zinc was first produced in India sometime in the 1400s.
|
https://d1ymz67w5raq8g.cloudfront.net/Pictures/480xAny/8/5/7/110857_the-elements_250_tcm18-209765.jpg
|
https://education.jlab.org/itselemental/ele030.html
|
View
|
|
25
|
Tb
|

|
Terbium
|
8.22
|
65
|
159
|
1629
|
3503
|
182
|
1.2
|
222
|
|
|
Hexagonal close-packed (hcp)
|
Silvery white
|
Lanthanide
|
Paramagnetic
|
Carl Gustaf Mosander (1843)
|
Terbium is a member of the lanthanide or rare earth group of elements. The silver-gray metal, which is relatively stable in air, is malleable and can be cut with a knife. Two crystal modifications are known.
|
https://lh3.googleusercontent.com/proxy/xMRFaQP_kUU-9qK6zeiZZnc4MzxLtTjnLzxBPVNeykAT9M0Tn1QmT6cevTL3mWA3aTDg8ITdaVMxwpmTDIoq8FFTpMSTANdW8S0ehArSKy7mWPJF9hYSL-wAyQ
|
https://www.livescience.com/38251-terbium.html
|
View
|
|
26
|
Pr
|

|
Praseodymium
|
6.64
|
59
|
141
|
1204
|
3563
|
193
|
1.13
|
|
|
|
Simple hexagonal
|
Silver
|
Lanthanide
|
Paramagnetic
|
Carl Auer von Welsbach (1885)
|
Praseodymium was named using the Greek words prasios didymos meaning ‘green twin,’ reflecting its green salts and the close association with neodymium. Pure metallic praseodymium was first produced in 1931.
|
https://hastingstechmetals.com/wp-content/uploads/2018/01/praseodymium.jpg
|
https://www.umicore.com/en/about/our-metals/praseodymium/
|
View
|
|
27
|
Tm
|

|
Thulium
|
9.32
|
69
|
169
|
1818
|
2223
|
160
|
1.25
|
|
|
|
Hexagonal close-packed (hcp)
|
Silvery gray
|
Lanthanide
|
Paramagnetic
|
Per Teodor Cleve (1879)
|
Thulium emits blue light upon excitation, a property that is exploited in flat panel display screens. Thulium also has useful applications in low-levelradiation detection and other luminescence applications, such as halide discharge lamps.
|
https://i0.wp.com/periodic-table.com/wp-content/uploads/2019/01/Thulium.jpg?fit=248%2C203&ssl=1
|
https://www.americanelements.com/tm.html
|
View
|
|
28
|
Ho
|

|
Holmium
|
8.79
|
67
|
164
|
1747
|
2973
|
165
|
1.23
|
20
|
|
|
Hexagonal close-packed (hcp)
|
Silvery white
|
Lanthanide
|
Paramagnetic
|
Jacques-Louis Soret and Marc Delafontaine (1878)
|
Holmium is never found in its pure form in the wild! Holmium oxide (Holmia) shows two dramatically different colors: yellow under natural light and pink under fluorescent lighting! Holmium has medical applications.
|
https://images-of-elements.com/holmium-2.jpg
|
https://www.utoledo.edu/nsm/ic/elements/holmium.html
|
View
|
|
29
|
Eu
|

|
Europium
|
5.264
|
63
|
152
|
1095
|
1800
|
182
|
1.2
|
|
|
|
Body-centered cubic (bcc)
|
Silvery white, with a pale yellow tint
|
Lanthanide
|
Paramagnetic
|
Eugène-Anatole Demarçay (1896, 1901)
|
The first, somewhat furtive, signal from element 63 was recorded in 1885 by Sir William Crookes, who found an anomalous red line (609 nm) in the emission spectrum of a samarium sample.
|
https://media.sciencephoto.com/image/c0266678/800wm
|
https://www.nature.com/articles/nchem.760
|
View
|
|
30
|
Er
|

|
Erbium
|
9.066
|
68
|
167
|
1770
|
3141
|
168
|
1.24
|
32
|
|
|
Hexagonal close-packed (hcp)
|
Silvery white
|
Lanthanide
|
Paramagnetic
|
Carl Gustaf Mosander (1843)
|
Erbium is a chemical element with the symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare earth element.
|
https://upload.wikimedia.org/wikipedia/commons/thumb/1/12/Erbium-crop.jpg/170px-Erbium-crop.jpg
|
https://en.wikipedia.org/wiki/Erbium
|
View
|
|
31
|
Yb
|

|
Ytterbium
|
6.57
|
70
|
173
|
1196
|
1469
|
154
|
1.1
|
|
|
|
Face-centered cubic (fcc)
|
Silvery white
|
Lanthanide
|
Paramagnetic
|
Jean Charles Galissard de Marignac (1878)
|
A soft, silvery metal. It slowly oxidises in air, forming a protective surface layer. Ytterbium is beginning to find a variety of uses, such as in memory devices and tuneable lasers. It can also be used as an industrial catalyst.
|
https://image.made-in-china.com/2f0j00jdOEGtcHZuos/Factory-Price-Rare-Earth-Ytterbium-Metal-Yb-Supplying.jpg
|
https://www.rsc.org/periodic-table/element/70/ytterbium
|
View
|
|
32
|
Pr
|

|
Promethium
|
7.26
|
61
|
145
|
1373
|
3273
|
|
1.13
|
|
|
|
Double hexagonal close-packed (dhcp)
|
Silver
|
Lanthanide
|
Paramagnetic
|
Chien Shiung Wu, Emilio Segrè, Hans Bethe (1945)
|
Promethium is a rare-earth metal that emits beta radius. It is very radoiactive and rare, so it is little studied: its chemical and physical properties are not well defined. Promethium salts have a pink or red colour.
|
https://www.raremetalbuy.com/wp-content/uploads/2016/07/Promethium.jpg
|
https://www.lenntech.com/periodic/elements/pm.htm
|
View
|






























