Magnetism around us - Properties
Aluminium Nickel Cobalt (AlNiCo)

Img src
All the alnicos are hard and brittle, much too brittle to be cold worked. (Hot working is possible but is not done commercially.) All production is therefore by casting of the liquid alloy or by pressing and sintering metal powders. The cast alloys have very coarse grains, of the order of 1 mm diameter. The sintered alloys are finer grained and mechanically stronger, with better surface finish but with somewhat inferior magnetic properties. They are usually limited to small magnets with cylindrical symmetry, for which the pressing operation is well suited. Surface grinding is the only finishing operation possible on either type of alnico.
The permanent magnetic properties of as-cast or as-sintered alnico are poor. A special, three-stage heat treatment is necessary to produce optimum properties:
- Heat to 1250oC for a time sufficient to produce a homogeneous solid solution.
- Cool at a rate of the order of 18C/sec to about 500oC or lower.
- Reheat (temper) at 600oC for a few hours.
Most alnicos are cooled (step 2 above) in a magnetic field of 1 kOe or more, or held in a magnetic field for 10–20 min at a temperature in this range. The field treatment increases the remanence measured in the direction of the applied field, and may slightly increase the coercive field. The final reheat (step 3) is not done in an applied field.
The alnicos achieve their permanent magnet properties by the precipitation of a ferromagnetic phase in a weakly magnetic matrix. Both phases are body centered cubic, and the phase separation occurs by spinodal decomposition, which results in a very regular array of ferromagnetic rods about 300A in diameter lying along <100> directions.
Applying a strong magnetic field during the precipitation process causes the rods to form preferentially along the [100] direction most nearly parallel to the field, rather than equally along all three possible <100> directions. Figure shows the remarkable degree of alignment and uniformity that can be attained in a single crystal.
Article src: Introduction to Magnetic Materials (2nd Edition), B. D. Cullity, C. D. Graham, Wiley-IEEE Press, 2008.
Iron-Silicon Alloys
Img src
In electrical power generation and transmission the greatest demand is for transformer cores. In this area silicon-iron is used to the exclusion of all others. This is also known as 'electrical steel' or silicon steel both of which are misnomers since these materials are not really steels.
In the power industry the electrical voltage is almost always low-frequency a.c. at 50 or 60 Hz. This leads to an alternating flux in the cores of the electromagnetic devices and consequently to the generation of 'eddy currents' if the material is an electrical conductor. Eddy currents reduce the efficiency of transformers because some of the energy is lost through eddy current dissipation.
There are several ways in which the properties of pure iron can be improved in order to make it more suitable for transformer cores at low frequencies. First the resistivity can be increased so that the eddy current losses become less. This is achieved by the alloying of silicon with iron. Iron containing 3% silicon has four times the resistivity of pure iron. Over the years there have been substantial improvements in the core losses of silicon iron.
Silicon of course is a very cheap material which is an important consideration when so much transformer iron is needed. It has two main beneficial effects on the properties of the alloy. The conductivity is reduced as silicon is added and the magnetostriction is reduced. For a.c. applications this reduction of magnetostriction is an additional advantage since the cyclic stresses resulting from magnetostrictive strains at 50 or 60 Hz produce acoustic noise.
Therefore any reduction of magnetostriction is desirable, particularly if it arises as a result of modifying the material to suit other unrelated requirements. A third benefit caused by the addition of silicon is that it reduces the anisotropy of the alloy leading to an increase in permeability in the non-oriented silicon-iron.
It is also beneficial to laminate the cores in such a way that the laminations run parallel to the magnetic field direction. This does not interfere with the magnetic flux path but does reduce the eddy current losses, by only allowing the eddy currents to exist in a narrow layer of material. Furthermore the coating of laminations with an insulating material also improves the eddy current losses by preventing current passing from one layer to the next. The thickness of the laminations is comparable with the skin depth at 50 or 60 Hz, which is typically 0.3-0.7mm, for optimum performance.
Article src: Introduction to Magnetism and Magnetic Materials (1nd edition), David Jiles, Chapman & Hall/CRC, 1991.
Iron–Cobalt Alloys
Img src
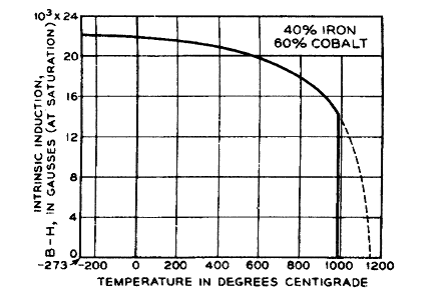
Cobalt is the only alloying element that substantially increases the Curie temperature and the saturation magnetization of iron. The alloys from about 30 to 50% Co all have room temperature saturation magnetization about 10% higher than iron, and Curie temperatures limited by a bcc to fcc phase transformation just under 1000oC. The 50–50 alloy, sold under various names, has low anisotropy and relatively high permeability, but quickly develops long-range order which makes it brittle. The addition of 2% V slows the ordering and allows the resulting alloy to be rolled into sheet form after rapid cooling from above the ordering temperature. Lower cobalt content alloys have less desirable soft magnetic properties, but cost less. The Fe–Co alloys are used where the highest saturation magnetization and/or a high Curie point is important: in the pole pieces of electromagnets, in beam-focusing lenses for electron microscopes, and in aircraft motors, generators, and transformers operating usually at 400 Hz.
The room-temperature maximum saturation of the binary cobalt-iron alloys occurs at a composition containing 35% Co. Because of the high H field needed to saturate (17,000 Gauss) for the measurement, the appropriate saturation is given as the intrinsic saturation or B-H instead of the usual B value. This composition corresponds closely to the Fe2Co found in the earliest studies. With further refinements, Williams (1915) was able to reach a value of 25,800 Gauss.
Article src: Article src: Introduction to Magnetic Materials (2nd Edition), B. D. Cullity, C. D. Graham, Wiley-IEEE Press, 2008.
Article src: Handbook of modern ferromagnetic materials, A. Goldman, B.S., Springer Science+Business Media New York, Ferrite Technology Worldwide, (1st edition), 1999.
Neodymium Iron Boron Magnets (FeNdB)
Img src
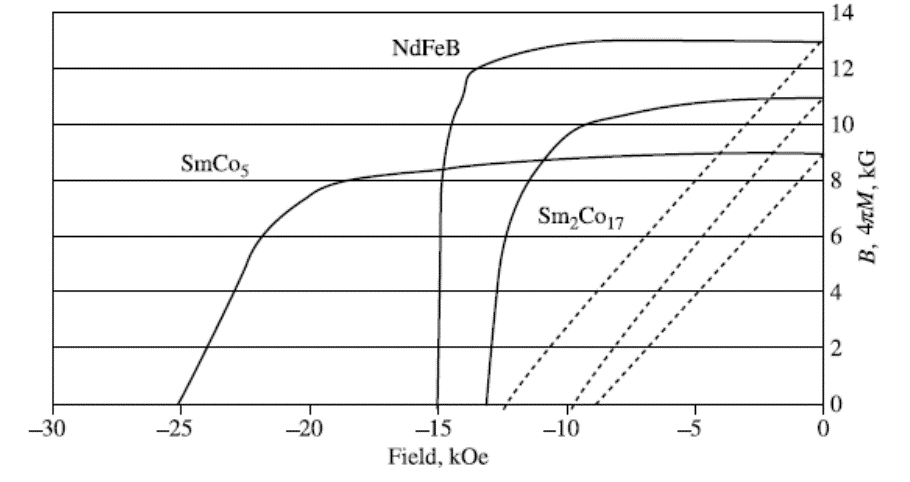
The principal disadvantages of the FeNdB family are a relatively low Curie temperature, near 300oC, which means a fairly strong temperature dependence of magnetic properties at room temperature, and susceptibility to severe corrosion in moist atmospheres. The corrosion problem is largely overcome with various metallic and nonmetallic coatings. The properties of examples of the three kinds of rare-earth permanent magnet materials are shown in Figure.
Magnets today (except alnico) can be made and used in the form of disks or plates magnetized through the thickness, because the coercivity is large enough to resist the large demagnetizing fields associated with such shapes. Alnico magnets need to be magnetized along their long dimension, so that the demagnetizing field is relatively small. Stated in another way, the permeance coefficient Bm/Hm at the (BH)max point is about 1 for barium ferrite or rare-earth magnets, compared to about 20 for Alnico 5. The rare-earth magnets are clearly far superior to either alnico or ferrite, and would replace them entirely except for their high cost.
An important variable not shown by the demagnetizing curve is the Curie temperature, which largely controls the temperature dependence of magnetic properties near room temperature. Alnico has the highest Curie temperature, and therefore the lowest temperature coefficient of Br.
Article src: Introduction to Magnetic Materials (2nd Edition), B. D. Cullity, C. D. Graham, Wiley-IEEE Press, 2008.
Permalloys

Img src
The soft magnetic properties of all the permalloys can be improved, in some cases dramatically, by a long high-temperature (1000oC) treatment in hydrogen to remove impurities such as C and S. This is a costly process, rarely used for commercial material. The electrical resistivity is increased, and heat treatment made less critical, by small additions of nonmagnetic elements, usually Mo, Cu, and/or Cr.
The 50 permalloy alloys have higher saturation magnetization, but the 78 permalloys have higher permeability and lower coercive field. Similar but not identical materials of both families are made under various trade names. As fiber optics have replaced copper in long-distance telephony, the production of permalloy, as well as the number of manufacturers, has decreased. However, a large number of uses remain, including ground fault interrupters (which protect people from electrical shock) and magnetic shielding.
The cube texture is fairly easy to produce in 50 permalloys by primary recrystallization after heavy cold rolling. The result is a square hysteresis loop, and such materials are produced under a variety of trade names.
A constant value of permeability over some range of applied field is a requirement for some specialized applications. An alloy called Isoperm was developed in Germany by making a cube-textured 50 Fe–50 Ni alloy and then cold rolling it to a 50% reduction in thickness. Along the hard-axis rolling direction the permeability is low (100 or less) and constant. A range of Ni–Fe–Co ternary alloys, of which the original was 25 Co, 45 Ni, 30 Fe, develop relatively strong anisotropy when annealed in a magnetic field. When these alloys are annealed in the demagnetized state, the domain walls are strongly pinned. Over the range of H and M where the wall motion is reversible, the permeability is almost constant and reasonably high, approaching a value of 1000. These alloys are called Perminvars, for invariant permeability.
Article src: Article src: Introduction to Magnetic Materials (2nd Edition), B. D. Cullity, C. D. Graham, Wiley-IEEE Press, 2008.
Samarium Cobalt (SmCo5)

Img src
SmCo5 magnets have an unusual and useful property: they can be magnetized initially by a field much smaller than their intrinsic coercive field Hci. This behavior is illustrated in Figure. The rule of thumb for permanent magnets is that the field required to satisfactorily magnetize the material is several times larger than the coercive field; this rule clearly does not apply to SmCo5. This behavior is a distinct production advantage, since reaching fields several times larger than the coercive field is difficult and expensive.
This phenomenon of easy magnetization but difficult demagnetization is interpreted to mean that the as-prepared magnet grains contain domain walls that move relatively easily in an applied field; this allows the magnet to be magnetized to saturation in relatively low fields. Once the grains have been magnetically saturated, and the domain walls driven out, reversing the magnetization requires the nucleation of new reverse domains, and there is a strong barrier to this nucleation. When the nucleating field is reached, the field is high enough to drive the domain walls completely through the grains and into saturation in the opposite direction. A magnet that behaves in this way is said to show nucleation-controlled coercivity.
SmCo5 magnets were the first to attain an energy product of 20 MGOe (160 kJ/m3), and they continue to be made and used.
Article src: Introduction to Magnetic Materials (2nd Edition), B. D. Cullity, C. D. Graham, Wiley-IEEE Press, 2008.
Soft Ferrites

Img src
The magnetically soft ferrites first came into commercial production in 1948. Their many applications will be briefly examined here, as well as the methods of making ferrites and the effects of such variables as porosity and grain size on their magnetic properties.
The soft ferrites have a cubic crystal structure and the general formula MO . Fe2O3, where M is a divalent metal such as Mg, Mn, or Ni. Nonmagnetic Zn ferrite is often added to increase Ms and all the commercial ferrites are mixed ferrites (solid solutions of one ferrite in another). The densities of the pure ferrites are a little over 5 g/cm3, Curie points range from about 300 to 600oC, and Ms from about 100 to 500 emu/cm3 (100–500 kA/m). Almost all of them have <k111> easy directions of magnetization, low magnetocrystalline anisotropy, and low to moderate magnetostriction.
The grain size of commercial ferrites ranges from about 5 to 40 mm, and as is true of most sintered products, metallic or nonmetallic, they are not completely dense. The true density is the mass of all the atoms in the unit cell divided by the volume of the unit cell. The cell volume is in turn found from the cell dimensions measured by X-ray diffraction. The density determined in this way is sometimes called the “X-ray density.” The porosity in ferrites can range from about 1 to 50%; a more typical range is 5–25%. Figure shows how an increase in sintering temperature increases the grain size and decreases the porosity; the black areas are voids.
Permeability increases as the grains become larger and as the porosity decreases. The grain-size effect is the stronger of the two. Porosity at the grain boundaries is less damaging to the permeability than porosity within the grains, because boundary porosity causes less hindrance to domain wall motion.
Article src: Introduction to Magnetic Materials (2nd Edition), B. D. Cullity, C. D. Graham, Wiley-IEEE Press, 2008.
Soft Iron

Img src
The magnetic properties of pure iron can vary in maximum permeability from 5,000 in the commercial product to 350,000 for the highest laboratory value. The lowest coercive force is about .01 Oersteds. The reported maximum permeabilities of iron are shown in figure over a period of years. The magnetic properties are affected strongly by the the heat treatment according to the temperature, time and rate of cool especially in the 800- 900° C. range as we shall see later. Impurities such as carbon can affect the aging of the material. Coercive force and hysteresis loss of some specimens will increase 100% or more after holding at 100°C, for 200 hours and will change appreciably even at 25° C. This phenomenon is caused by precipitation of C or N if their solubilities are exceeded. The solubilities of some impurities in iron are affected by the presence of others. Manganese reduces the solubility of sulfur and carbon reduces the solubility of silicon. Other elements such as Ti and V counteract the effect of aging.
Hydrogen annealing by Cioffi (1932,1934, 1937) got a maximum permeability of 250,000 after a long moist hydrogen anneal and going through the a-γ transformation in several hours. While good D.C. properties can be achieved in pure iron, the drastic heat treatment required is too expensive to be applied in many commercial applications.
Article src: Handbook of modern ferromagnetic materials, A. Goldman, B.S., Springer Science+Business Media New York, Ferrite Technology Worldwide, (1st edition), 1999.