Down to nanoscale - Applications
- Biomagnetic separation
- Magnetic catalysis
- Magnetic gene delivery
- Magnetic nanodiamond particles
- Magnetic nanoparticles as catalysts
- Magnetic nanostructures
- Magnetic Particle Hyperthermia
- Magnetic resonance imaging (MRI)
- Magnetic Sensors and detectors
- Magnetic storage (Hard drives)
- Magnetic water purification
- Nanowires
Biomagnetic separation
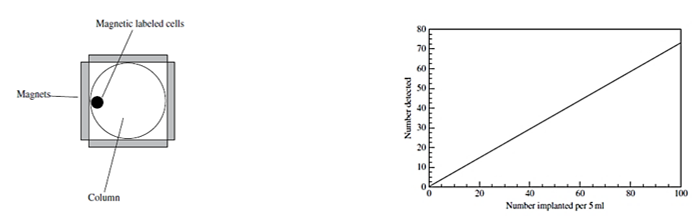
Figure right Plot of the number of cells detected by the device in Figure 1 versus the Number intentionally added to the bloodstream.
Img src
Ferrofluids can be used to both detect the presence of tumor cells in the blood and remove them. The presence of tumor cells in the blood at certain levels is an indication of the possibility of cancer spreading called metastasis. The cells in the blood can be loaded with magnetic nanoparticles by inducing collisions between the cells and the nanoparticles. This can be accomplished by injecting a ferrofluid into the bloodstream and applying a force to the particles using an external magnetic field having a gradient.
The presence of the magnetic labeled tumor cells can be detected by passing the cells through a column surrounded by magnets as illustrated in Figure 1. As the blood flows through the column, the cells containing the magnetic particles are attracted to the magnets and stick to the walls of the column. This method can be used to detect tumor cells and remove them from the blood. The tumor cells on the walls can be detected by various spectroscopic methods such as atomic force microscopy and magnetometry.
Article src: Physics of Magnetic Nanostructures, First Edition. Frank J. Owens, Inc. Published by John Wiley & Sons, Inc., 2015.
Magnetic catalysis
Img src
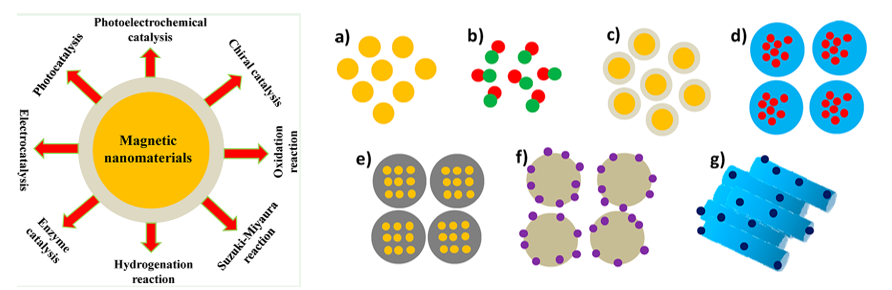
Magnetic nanomaterials show promising applications in heterogeneous catalysis because of their ease of separation and good reusability. The magnetization saturation values of magnetic nanoparticles mainly depend on finite-size and surface effects. For small magnetic nanoparticles, the formation of domain walls provides a high energy state and the particles aggregate easily in clusters or clumps. To avoid agglomeration, magnetic nanoparticles can be coated with different shells (e.g., silica, carbon, metal, metal oxide, and polymer) to isolate them from external environments. Because of high surface area of magnetic nanoparticles, many active species can be supported on the surface to enhance the catalytic activity.
Many organic compounds are important chemical reagents and intermediates in organic synthesis. To date, many catalytic systems have been developed to facilitate the conversion of reactants to target products. Despite high efficiency, homogeneous catalysts exhibit difficulty in separation, which limits their large-scale applications. Different magnetic nanocomposites, including spherical, bimetallic, typical core−shell MNPs, multinuclear MNPs inlaid in core−shell structures, MNP−silica nanocomposites, MNP-decorated silica spheres, and MNP encapsulated in mesoporous materials, can be used to catalyze different reactions (Figure). Magnetic metal oxides (such as Fe3O4, MnFe2O4, and CoFe2O4 ) and metal alloys (such as CoPt3 and FePt) possess specific magnetic properties measured by the magnetization cycle in a hysteresis loop and have been successfully applied in heterogeneous catalysis. Additionally, hybrid MNPs have currently attracted a great deal of attention because of the functionalization of materials that can be used as suitable supports with high surface area to immobilize active species, and can be coated by C, silica, metal, metal oxide, and polymer to form a core@shell structure.
Article src: ACS Appl. Nano Mater. 2019, 2, 4681−4697
Magnetic gene delivery

Img src
Gene delivery is an indispensable technique for various biomedical applications such as gene therapy, stem cell engineering and gene editing. Recently, magnetic nanoparticles (MNPs) have received increasing attention for their use in promoting gene delivery efficiency. Under magnetic attraction, gene delivery efficiency using viral or nonviral gene carriers could be universally enhanced. Besides, magnetic nanoparticles could be utilized in magnetic resonance imaging or magnetic hyperthermia therapy, Attempts have been made to incorporate magnetic nanoparticles
(MNPs) with both viral and nonviral gene carriers. An external magnetic field (EMF) could be applied to significantly promote the cellular uptake of MNP-containing gene vectors in vitro and improve the delivery efficiency to targeted tissues/organs in vivo. Superparamagnetic iron oxide (SPIO) nanoparticles, which possess good biocompatibility and superparamagnetism, are ideal materials to prepare MNP-based gene carriers. Compared to other gene delivery systems, MNPs possess unique advantages
for their rapid transfection process, magnetic targeting, isolation/positioning of transfected cells and molecular imaging. With EMF
application, MNPs could accelerate gene transfection kinetics and elevate gene shuffling into the cells. The reduction of transfection doses and time by MNPs contributes to fewer side effects from the overexposure of gene delivery vectors providing extra theranostic opportunities.
Article src: J. Mater. Chem. B, 2021, 9, 4267–4286
Magnetic nanodiamond particles

Img src
Nanodiamonds are carbon nanoparticles with truncated octahedral architecture that are about 2–8nm in diameter and can deliver a wide range of therapeutics, including small molecules, proteins, and nucleic acids. Among other nanoparticle systems, nanodiamonds (NDs) appear to be a promising candidate for the multifunctional applications due to their surface, electrochemical, and optical properties; and the availability in various sizes and surface structure, and, most importantly, the demonstrated biocompatibility. Magnetic NDs (MNDs) can combine properties and advantages of the ND and magnetic nanoparticles. The most developed and clear way to prepare magnetic ND is doping with magnetic atoms. However, in some cases, such NDs can show some level of cytotoxicity and be not biocompatible. As for magnetic properties of diamond in general, pure diamond structure combines diamagnetic and paramagnetic components, but in an intermediate graphite–diamond structure the conditions for spin ordering and magnetic interactions can be created. Along with the soft ferromagnetism of ND, the increased fluorescence at one and two-photon excitation may be realized. Utilizing the combined magnetic and fluorescence properties of the magnetically modified NDs, fluorescence imaging, fluorescence lifetime imaging and manipulation of cells by magnetic field may be demonstrated. Thus, novel perspectives to use the magnetic ND for drug delivery, cells magnetic separation and filtration, in bioengineering to control the cell distribution combined with imaging and treatment are opened.
Article src: DOI: 10.1117/1.JBO.23.9.091404, Doi: https://doi.org/10.1038/nnano.2011.209
Magnetic nanoparticles as catalysts

Img src
Catalysis involves the modification of the rate of a chemical reaction, usually a speeding up or acceleration of the reaction rate, by the addition of a substance called a catalyst that is not consumed during the reaction. Ordinarily, the catalyst participates in the reaction by combining with one or more of the reactants, and at the end of the process, it is regenerated without change. In other words, the catalyst is being constantly recycled as the reaction progresses. There are two main types of catalysts. Homogeneous catalysts are dispersed in the same phase as the reactants, the dispersal ordinarily being in a gas or a liquid solution. Heterogeneous catalysts are in a different phase than the reactants, separated from them by a phase boundary. Heterogeneous catalytic reactions usually take place on the surface of a solid catalyst, such as silica or alumina, which have very high surface areas that typically arises from their porous or spongelike structure. These catalysts have their surfaces impregnated with acid sites or coated with a catalytically active material such as platinum, and the rate of the reaction tends to be proportional to the accessible area of a platinum coated surface.
The chemical activity of a heterogeneous particle catalyst is proportional to the ratio of the surface area to the volume, A/V. Since nanoparticles have a high A/V ratio, they have the potential to be good catalysts. However, magnetic nanoparticles that can also be catalysts can be an exception to this rule. Rhodium, is paramagnetic in the bulk, becomes ferromagnetic when it has nanometer dimensions. Figure shows the effect of rhodium nanoparticles on the dissociation of carbon monoxide.
Article src: Physics of Magnetic Nanostructures, First Edition. F. J. Owens, Inc. Published by John Wiley & Sons, Inc., 2015.
Magnetic nanostructures
Img src
Magnetic nanostructures have found a wide range of applications in information storage, drug delivery, magnetic hyperthermia, cell separation and magnetic refrigeration. Hard magnetic nanostructures are used for bonded magnet preparation, recording media, and microelectromechanical system (MEMS) applications. Self-assembled FePt nanoparticle arrays are potential candidates for high-density data storage. Soft magnetic nanostructures are used in common-mode electrical chokes, high-frequency transformers, magnetic sensors, magnetic shielding sheets, and for flux guidance in permanent magnets.
Nanostructuring is used to reduce magnetic losses by controlling anisotropy and eddy current losses. Magnetic nanoparticle-based magnetic relaxation switch (MRSw) biosensors have been used for nucleic acid, protein, enzyme, and virus sensing. When magnetic nanoparticles interact with molecular targets, they switch from dispersed state to clustered (or the reverse); this changes the magnetic resonance of the solvent. This change of magnetic relaxation can be detected to characterize the attached molecular targets.
Article src: Handbook of Nanomaterials, R. Vajtai, Springer-Verlag Berlin Heidelberg, 2013.
Magnetic Particle Hyperthermia
Img src
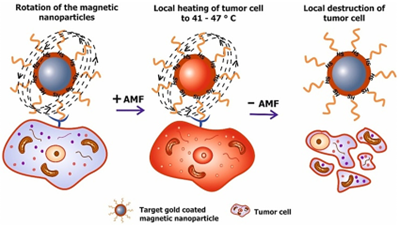
Magnetic Particle Hyperthermia: MPH (alternatively named as magnetic fluid hyperthermia: MFH) is the regional, magnetically originated, heat release due to the presence of MNPs in the malignant region. First attempts, to incorporate macroscopic magnetic implants as heat generators, goes back in the 1950s while gradually, nanoscale entities such as MNPs are up to date routinely used to deliver heat cargo at specific sites. MNPs generate heat in an alternating magnetic field as a result of hysteresis and/or relaxational losses (Néel/Brown), resulting in heating of the tissue in which MNPs are accumulated. With the development of precise methods for synthesizing functionalized MNPs, MNPs with functionalized surfaces, which have high tumor specificity, have been developed as heating elements for magnetic hyperthermia. Furthermore, there is renewed interest in magnetic hyperthermia as a treatment modality for cancer, especially when it is combined with other more traditional therapeutic approaches such as the co-delivery of anticancer drugs or photodynamic therapy. The current development of magnetic hyperthermia is heavily focused on two aspects, namely the composition of the nanoparticles (where reproducibility and scalability are consistently found to be hard to achieve), and the instrumentation needed for applying external fields to generate the magnetic hyperthermia, and for measuring the resultant heat deposition in tissues. Regarding the instrumentation, the clear majority of the devices are purpose- built designs intended for in vitro testing or at most pre- clinical in vivo testing. Clinical scale appliances are very much the exception to date and are being developed by companies such as Magforce GmbH in Berlin and Resonant Circuits Ltd in London. The incorporation of MNPs in hyperthermia protocols has many advantages. First, it is a mild, least-invasive scheme since the frequencies and intensities of magnetic fields generally utilized pass harmlessly through the body. Additionally, its performance is effectively and externally guided, while its effect may be delivered down to the cellular level. Second, MNPs can be targeted through cancer-specific binding agents making the treatment much more selective and effective. Since MNPs are only a few tens of nanometer in size, they may find easy passage into several tumors whose pore sizes are in 380–780 nm range. Moreover, specific MNPs can also effectively cross blood–brain barrier (BBB) and, hence, can be used for treating brain tumors. Third, with the possibility to obtain stable colloids using MNPs, they can be administered through several drug delivery routes. Compared to macroscopic implants, MNPs provide much more efficient and homogeneous treatment. Fourth, MNP-based hyperthermia treatment may induce antitumoral immunity while it may appear as a branch of multi- therapeutic approaches for treating many diseases. For example, a multifunctional combination of MPH with other assisting toxic agents (mostly irradiation or chemotherapy drugs) often succeeds in reliable damage of tumor cells where isolated treatments are proved inefficient.
Article src: Magnetic Particle hyperthermia, M. Angelakeris, Book Chapter in Vol.8 Nanopharmaceuticals, Nanomedicine, and Food Nanoscience of 21st Century Nanoscience, A handbook, (10 volumes), Taylor & Francis, (2020).Magnetic resonance imaging (MRI)
Img src
Magnetic resonance imaging (MRI) is undoubtedly the most important and best-established clinical application of magnetism in medicine. Three Nobel prizes have been awarded for it, in chemistry and medicine. There are about 20 000 MRI scanners installed in hospitals worldwide, and roughly 100 million examinations are carried out every year. The technique combines NMR with sophisticated signal processing to yield two-dimensional tomographic images or three-dimensional views of a solid object, usually a part of the human body. The analysis is based on the 1H resonance. Radio-frequency radiation is absorbed at a frequency corresponding to the nuclear Zeeman splitting of protons in the body. Fortunately, some 63% of the atoms in the human body are hydrogen, mainly in fat and water.
The instrument consists of a magnet, shimmed to produce a highly uniform magnetic field, a set of three-dimensional gradient coils and radio-frequency coils to excite and detect the resonace. The magnets are usually superconducting solenoids, cooled with liquid helium or a cryocooler, which generate fields of 1.0–1.5 T. Higher fields of 3 T or more provide improved resolution and contrast. Permanent magnet systems are simpler, but bulky and limited to fields of about 0.4 T.
Magnetic resonance imaging (RI) contrast agents are categorised according to the following specific features: chemical composition including the presence or absence of metal atoms, route of administration, magnetic properties, effect on the magnetic resonance image, biodistribution and imaging applications. The majority of these agents are either paramagnetic ion complexes or superparamagnetic magnetite particles and contain lanthanide elements such as gadolinium (Gd3+) or transition metal manganese (Mn2+). These elements shorten the T1 or T2 relaxation time, thereby causing increased signal intensity on T1-weighted images or reduced signal intensity on T2-weighted images.
Article src: 10.3892/ijmm.2016.2744
Magnetic Sensors and detectors
Img src
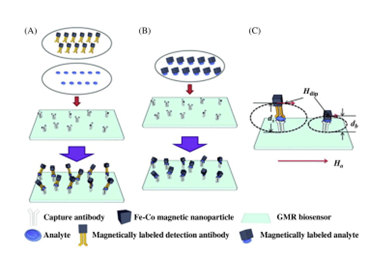
Nanosensors are any biological, chemical, or surgical sensory points used to convey information about nanoparticles to the macroscopic world. Their uses mainly include various medical purposes and gateways to build other nanoproducts, such as computer chips that work at the nanoscale and nanorobots. One of the first working examples of a synthetic nanosensor was built by researchers at the Georgia Institute of Technology in 1999. It involved attaching a single particle onto the end of a carbon nanotube and measuring the vibrational frequency of the nanotube both with and without the particle. Recently, a large number of works were reported with seeking of developing magnetic nanoparticles (MNPs) because of their specific benefits, for example, their low cost of synthesis, physicochemical characteristics, and sizes. MNPs show their topic performances at 10-20 nm in size because of their supermagnetic behavior that makes them particularly appropriate to obtain quick responses under applied magnetic fields. Besides, MNPs exhibit high mass transference and wide surface area. The synthesis of MNPs must be designed with the aim of obtaining NPs with suitable sizes-dependent physicochemical characteristics. MNPs having controlled surface and appropriate physicochemical properties have been prepared in specific circumstances for a numerous applications involving biosensors, sensors, tissue engineering, imaging, cell labeling, magnetic hyperthermia, magnetic resonance imaging, disease therapy, water purification, wastewater treatment, and drug delivery. In addition, MNPs are found to be useful to develop the stability and sensitivity of sensor devices for detecting numerous analytes in environmental, food, and clinical applications. Sensing approaches based on MNPs provide benefits in terms of analytical figures of merit, for example, rapid analysis time, high ratio of signal to noise, low detection limit (DL), and improved sensitivity compared to non-MNPs-based technologies. MNPs are employed via direct applications of labeled supports to the sensors, inserted inside the transducers. The MNPs are distributed into the material by attracting them using an externally magnetic field on the active surface of detection of the sensors or biosensors. MNPs-based biosensors and sensors are founded on magnetic field, piezoelectric, optical, and EC as transduction principles. Magneto-resistive sensors initiate by the fundamental magneto-resistance of ferromagnetic materials or on nonmagnetic/ferromagnetic hetero-structures. The analytical signal, which has a variation in electrical resistance, is determined ensuing the analytes binding under an externally magnetic field. Consequently, the analytical signals could be acquired by feeble variations in magnetic fields and varies with the applied magnetic fields beside the area of nanosensor as shown in the figure. For better magneto-resistive detection the quality of the MNPs will be very essential. Therefore perfect probes would be SPM and exhibiting high susceptibility and large magnetic moments, which permit their magnetizations under small magnetic fields.
Article src: https://doi.org/10.1016/B978-0-12-819870-4.00009-8
Magnetic storage (Hard drives)
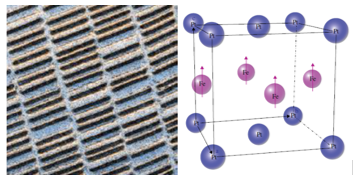
Figure right: Tetragonal unit cell of FePt. Arrows on the iron atoms indicate direction of Magnetic moments in the ferromagnetic phase.
Img src
The study of magnetic materials, particularly of films made of nanomagnets, is driven by the desire to increase storage space on magnetic storage devices such as hard drives in computers. The basic information storage mechanism involves alignment of the magnetization in one direction of a very small region on the magnetic tape called a bit. To achieve a storage of 10 gigabits per square inch, a single bit would be approximately 1 μm long and 70 nm wide. The film thickness could be about 30 nm. Existing magnetic storage devices such as hard drives are based on single layers of nanosized crystals of cobalt chromium alloys such as CoPtCrTa. Left figure shows a magnetic force microscope image of a typical hard drive layer.
There is an intense research effort to increase the storage capacity of hard drives. In order to achieve this, smaller stable nanoparticles having high coercivity, low magnetization, and negligible magnetic coupling between the particles are required.
The interaction between the particles can be reduced by coating them with a nonmagnetic material. The effort to reduce the particle size of the CoPtCrTa alloys in order to increase storage capability has resulted in particle sizes down to 8–10 nm. This is close to the size where the particles become superparamagnetic. At this size, the thermal energy at room temperature, kT, is close to the magnetic anisotropic energy, which means the particles can undergo thermal fluctuation and become disordered resulting in loss of stored information. Present efforts to increase storage capacity are focusing on identifying smaller particles that have higher magnetic anisotropic energies. One candidate material is FePt. It has a face‐centered tetragonal crystal structure illustrated in right figure. The structure consists of two‐dimensional layers of iron atoms (arrows in figure) between layers of Pt atoms. The easy direction of magnetic field‐induced alignment of the magnetic moments is parallel to the tetragonal C axis. FePt has a very large anisotropic constant, K, which is about 50–100 times larger than the CoPtCrTa alloys. This means much smaller particles in the order of 3.0 nm can be used on the layers in the hard drive.
Article src: Physics of Magnetic Nanostructures, First Edition. Frank J. Owens, Inc. Published by John Wiley & Sons, Inc., 2015.
Magnetic water purification
Img src
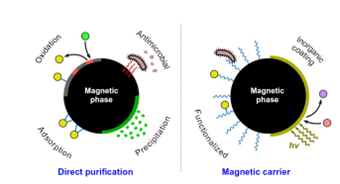
Water purification by nanoparticles is a rather challenging field of technology which is determined by a number of restrictions related to (i) the low concentrations of pollutants to be treated, (ii) the extremely low regulation limits that should be accomplished after treatment, and (iii) the need to ensure the absence of nanoparticles in purified water and waste streams. In general, a high uptake efficiency against inorganic (heavy metals) and organic (pesticides, microorganisms, pharmaceuticals) pollutants can be realized by case-specific inorganic nanoparticles which either work as the direct active phase (dispersed in water or attached on a solid matrix) or as a carrier onto which another active phase or molecule is loaded. However, beyond the attempt to develop optimized nanoparticles with high affinity to water pollutants, the separation of used nanoparticles from the purified water stream should be considered an equally important task of the process design. Suggestively, with respect to the large quantities of nanoparticles that should be removed in full-scale systems using particles dispersion, the cost for particles separation may be the dominant consideration if a typical nanofiltration step is applied. To overcome the issue of nanoparticles recovery after application in water purification, magnetic separation processes (i.e., magnetophoresis) present a more sophisticated way promising minimum
chemical or physical interaction with treated water, very low cost, total recovery of nanoparticles, and multiple options for the design of the process. Magnetophoresis is also favorable due to the fact that many of the accessible nanoadsorbents consist of phases with high magnetic response (iron oxides, zero-valent iron) whereas magnetic nanoparticles can be easily used as substrates for other active phases.
Article src: Nanoscale Materials in Water Purification. https://doi.org/10.1016/B978-0-12-813926-4.00026-4
Nanowires
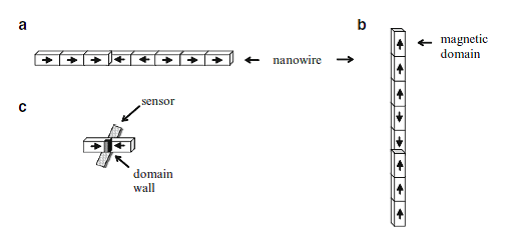
Img src
Interesting spintronic chips with racetrack memory made of magnetic domains within nanowires have become nowadays one of the latest storage devices to be comprehensively investigated. Nanowires lie horizontally or stand vertically on silicon substrates while magnetic domains contained in the nanowires store bits of data. The domains are moved rapidly along the tracks by pulses of electric current. Magnetic fields emanating from the domain wall set the magnetization direction in the racetrack’s bit. Thus, the domain wall separating two oppositely magnetized domains is used to write a data bit on the racetrack. While the magnetic domains pass a sensor lying underneath the nanowire, the changing magnetization between oppositely magnetized domains is recorded.
Because of this rapid displacement along the track past read/write heads placed at fixed locations, these information storing magnetic nanowires are known as “racetrack memory.” The magnetic domains are nonvolatile and rewritable, thereby comparable to HDD. Nevertheless, one of their big advantage over HDD is that the chip has no mechanical moving parts because only electrons read and write bits. This also makes them very fast, while not compromising on reliability.
Each magnetic domain represents a “1” or a “0” of stored data, and retains its data when power is switched off. The sensing layer of the magnetic head changes its magnetization back and forth in order to match the field of each domain passing it. Domain walls can move as fast as 150 nm/ns, allowing access millions of times faster than HDDs. With perpendicular columns of nanowires, large data densities become possible on one silicon chip, overcoming many limitations of their predecessor memories. As bits move rapidly along the racetrack, a new generation of data intensive applications is envisaged, especially since the magnetic heads themselves have evolved. From the spin valve sensors relying on GMR to the MTJs exploiting TMR (tunneling magnetoresistance) to achieve a greater sensitivity to small magnetic fields, new spintronic technologies come to the aid of magnetic recording. As such, the racetrack memories benefit from spintronics as well.
Article src: Magnetism: Basics and Applications, Stefanita, Carmen-Gabriela, Springer Heidelberg Dordrecht London New York, 2012.