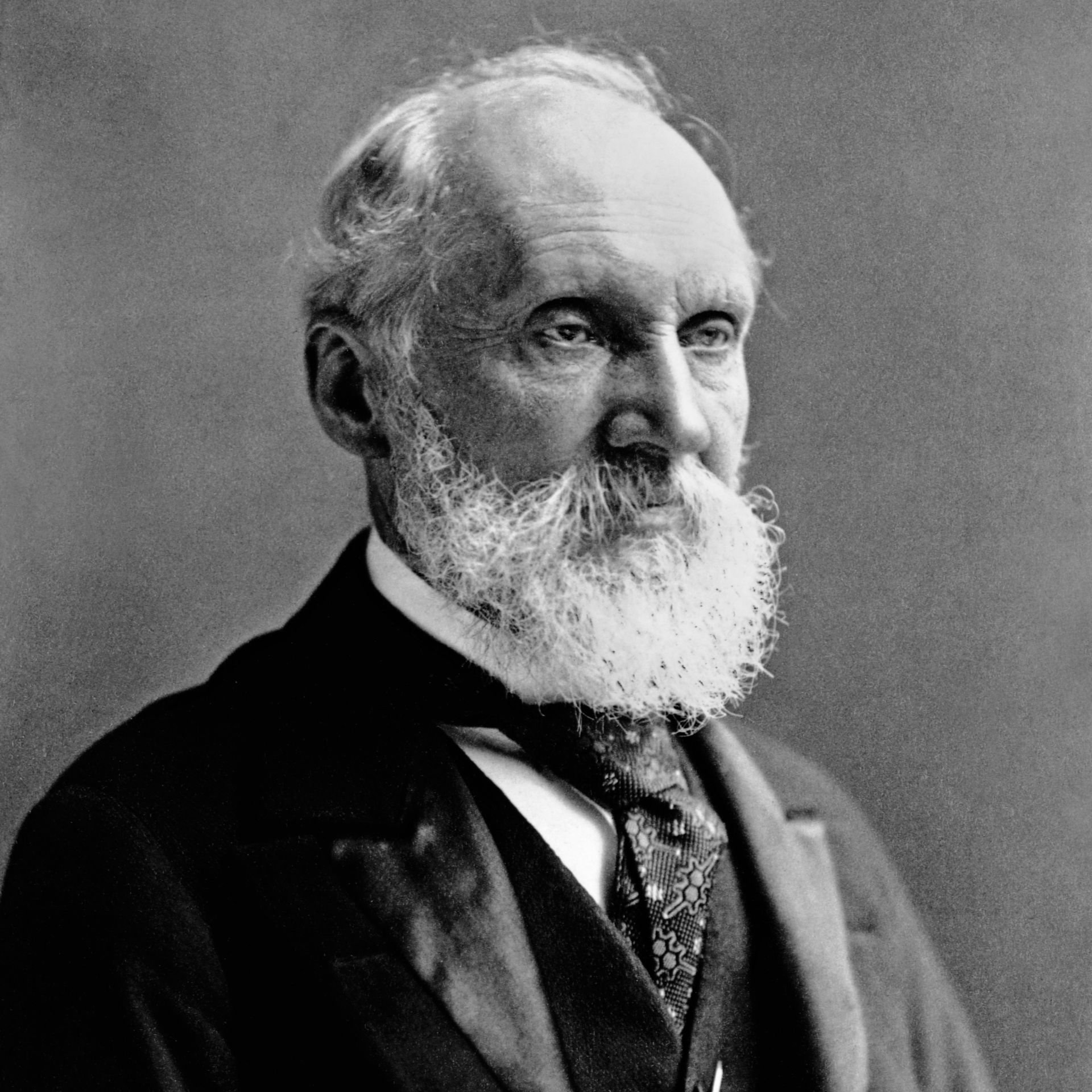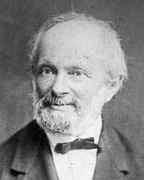It all started like this - Pioneers
Read more +
As a student, Rohrer was interested in physics, chemistry, and classical languages. He decided to concentrate on physics when he enrolled at the Swiss Federal Institute of Technology in Zurich in the autumn of 1951. One of his professors was Wolfgang Pauli, who won the Nobel Prize in Physics in 1945 for the discovery of the exclusion principle, better known as the Pauli principle. From Pauli and his other professors, Rorher learned the fundamentals of physics, and he received a bachelor of science degree from the Institute in 1955.
Conducted Graduate Work On Superconductors
In the autumn of 1955, Rohrer started work on his Ph.D. thesis on superconductivity. "It was fortuitous that Jörgen Lykke Olsen trusted me to measure the length changes of superconductors at the magnetic field-induced superconducting transition," Rohrer said in his autobiography posted on the Nobel Prize Website. "He had already pioneered the field with measurements on the discontinuity of Young's modulus. Following in his footsteps, I lost all respect for angstroms [an atomic measure]. The mechanical transducers were very vibration sensitive, and I learned to work after midnight, when the town was asleep." Rohrer recalled that his graduate years were fun and memorable, although they were interrupted by his basic training in the Swiss mountain infantry. He received his Ph.D. in experimental physics from the Institute in 1960, and for a year afterward worked as a research assistant at the institute.
In the summer of 1961, Rohrer married Rose-Marie Egger, whom he has credited with bringing a stabilizing influence into his life. The couple took their honeymoon in the United States, where Rohrer spent two years doing postdoctorate research at Rutgers University in New Brunswick, New Jersey, working on the thermal conductivity of type-II superconductors and metals.
Joined IBM In Switzerland
Following a four-month camping trip through the United States, Rohrer returned to Switzerland in 1963. In the summer of that year, Ambros Speiser, director of the newly founded IBM Research Laboratory in Ruschlikon, Switzerland, offered Rohrer a position at the company as a research staff assistant. Encouraged to accept this position by his professor Bruno Luthi, Rohrer joined IBM in December of 1963. His research efforts interests included Kondo systems, phase transitions, multicritical phenomena, scanning tunneling microscopy and, most recently, nanomechanics.
By the end of the decade, colleague Keith Blazey, who had done optic experiments on GdAlO3, an antiferromagnet, asked Rohrer to work with him. Thus began the collaboration on magnetic phase diagrams that would eventually lead Rohrer into the field of critical phenomena. K. Alex Muller, who won a share of the 1987 Nobel Prize in Physics for breakthroughs in the discovery of superconductivity in ceramic materials, had pioneered efforts in critical phenomena at IBM's Ruschlikon laboratory, and he encouraged Rohrer in this new direction. Roher focused on the bicritical and tetracritical behavior and on the random-field problem. "These were most enjoyable years, during which so many patient colleagues taught me physics," recalled Rohrer in his Nobel autobiography.
Rohrer remained involved with IBM throughout his career. "In all the years with IBM Research, I have especially appreciated the freedom to pursue the activities I found interesting, and greatly enjoyed the stimulus, collegial cooperation, frankness, and intellectual generosity of two scientific communities, namely, in superconductivity and critical phenomena," Rohrer wrote in his Nobel autobiography. Until he retired, Rohrer's only time away from the company came in the mid-1970s, when he took a year-long sabbatical to study nuclear magnetic resonance at the University of California, Santa Barbara. Curious about nuclear magnetic resonance, Rohrer began the sabbatical in 1974, working with professors Vince Jaccarino and Alan King. Together the three men solved a specific problem on the bicritical point of MnF2, their home-base material.
Began Collaboration With Binnig
Rohrer eventually became interested in the complex atomic structures of surface materials, structures that were then little understood. The development of the electron microscope had enabled investigation into the arrangements of atoms in materials, but attempts to discover information about the much different nature of surface atoms had met with little success.
In 1978 in Zurich, Rohrer began a fruitful collaboration with Gerd Binnig, a young German who had just completed his doctorate work. Rohrer and Binnig began exploring oxide layers on metal surfaces. To further their research, they decided to develop a spectroscopic probe. However, in the process, they designed a new type of microscope, one that would make it possible to study, in the greatest detail possible, the atomic structure of the surface being examined. Images of individual atoms on a metal or semiconductor surface would be formed by scanning the tip of a needle probe over the surface, at a height of only a few atomic diameters.
Development of this unique microscope began when Rohrer and Binnig used a technique called tunneling. Quantum mechanics had revealed that electrons behave in a wave-like fashion that causes them to create a spreading cloud as they are emitted from the surface of a sample. When the electron clouds from two close surfaces overlap, the electrons then "tunnel" from one cloud to the other. The technique of causing such tunneling through an insulating layer had been useful in revealing information about the atomic materials on either side of the insulation.
For their approach, Rohrer and Binnig tunneled through a vacuum and then used a needle-like probe inside the vacuum to scan the sample surface. As the tip of the probe neared the sample, the electron clouds of each overlapped and a tunneling current began to flow. The scientists employed a feedback mechanism that harnessed the current to keep the probe tip at a constant height above the sample surface, enabling the tip to follow the contours of the individual atoms of the scanned surface. A computer then processed the tip's motion and used the data to produce a three-dimensional, high-resolution image of that surface.
From the start, Rohrer and Binnig knew they were on to something with this innovative technique. However, they encountered problems, the first being that the probe tip was sensitive to disturbances from vibration and noise. Building upon Rohrer's previous experience with superconductors, where transducers experienced the same kind of sensitivity, Rohrer and Binnig decided to shield the probe from disturbances with magnets and a heavy stone table set on inflated rubber tires. This solved their problem. The basic device was successfully tested in 1981, and Rohrer and Binnig then refined it technologically. The resulting microscope was built on a heavy permanent magnet floating in a dish of superconducting lead. By the mid-1980s, all but the vacuum chamber of the scanning tunneling microscope could fit in the palm of one's hand and could show details as tiny as one-tenth of an angstrom. One angstrom is equivalent to the diameter of a single atom, or 2.5 billionths of an inch. Later, scanning tunneling microscopes were developed that would also work in water, air, and cryogenic fluids. In 1987, Rohrer's research group at IBM had developed a scanning tunneling microscope the size of a fingertip.
Won Nobel Prize
In 1986, Rohrer and Binnig received the Nobel Prize in Physics for inventing the scanning tunneling microscope. They shared the award with Ernst Ruska, who was recognized for his fundamental work in electron optics and for designing the first electron microscope. When awarding the Nobel Prize, the Royal Swedish Academy of Sciences said that the scanning tunneling microscope was a completely new device that was only at the beginning of its development. While acknowledging that the device had only been successfully tested for the first time in 1981, the Academy added: "It is, however, clear that entirely new fields are opening up for the study of the structure of matter. Binnig's and Rohrer's great achievement is that, starting from earlier work and ideas, they have succeeded in mastering the enormous experimental difficulties involved in building an instrument of the precision and stability required." As the Swedish Academy expected, the scanning tunneling miscroscope was soon used in fields as diverse as semiconductor science, metallurgy, electrochemistry, and molecular biology. More recently, it has proved to be an essential tool for the new science of nanotechnology.
Later Career
Besides their Nobel prize, Rohrer and Binnig also received the King Faisal Prize and the Hewlett Packard Europhysics Prize in 1984 for their invention of the scanning tunneling microscope. In 1987, Rohrer received the Cresson Medal of the Franklin Institute in Philadelphia. The invention of the miscroscope also led to Rohrer's induction into the U.S. National Inventors Hall of Fame in 1994. In addition, Rohrer has also been awarded honorary doctorates by several universities. Continuing his work following the Nobel award, Rohrer was appointed an IBM fellow in 1986, and he served as manager of the physical sciences department at the Zurich Research Laboratory from 1986 to 1988. He retired from IBM in July of 1997 and accepted research appointments at the Consejo Superior de Investigaciones Científicas in Madrid, Spain, and at Riken and Tohoku University in Japan.
The Small World Of Nanoscience
In more recent years, Rohrer's interest has focused on nanoscience and nanotechnology. Nanoscience involves particles that are smaller than atoms. A nanometer is one-billionth of a meter, or 1/1,000,000,000th of a meter. Five hydrogen atoms placed side by side would span about one nanometer. A single human cell encompasses thousands of nanometers.
At this level, scale is so small that generally accepted principles of physics no longer apply. Forces such as inertia, friction, and gravity act differently or are not even meaningful. Nanoscience tries to make sense of how matter behaves at this level, while nanotechnology involves research and technology development at the atomic, molecular or macromolecular levels; creating and using the structures, devices, and systems that have the properties needed to deal with such material; and the ability to control or manipulate on the atomic scale.
Rohrer believes the world should be ready to exploit the new possibilities nanotechnology represents. In addition, he feels that devices like the scanning tunneling microscope would be useful in addressing one of the biggest challenges of nanoscience and nanotechnology: improving the interface between the macroscopic world of traditional manufacturing and the "nano" world. The scanning tunneling microscope could help create such an interface and thus become one of the major tools of nanotechnology. Along with its imaging capabilities, the microscope could be reconfigured to manipulate molecules and atoms, and it would enable researchers and product developers to observe what happens at the molecular level, so that they could modify and manufacture items at the nano level.
Read more +
In 1911 it was discovered that certain metals, when cooled to low enough temperatures, can carry current with no resistance. This seemingly miraculous property, superconductivity, arises directly from quantum mechanics, and underlies many contemporary technologies, such as magnetic resonance imaging body scanners and particle accelerators. For decades, however, there was no theory to explain how electrons in superconducting materials overcome their own mutually repulsive properties and other causes of resistance.
In early 1957, Schrieffer, then a 25-year-old graduate student, wrote down a quantum-mechanical wave function that accounted for the behaviour of electrons in superconductors. With his thesis adviser John Bardeen and postdoc colleague Leon Cooper, he published the now-famous BCS wave function and the full theory of superconductivity less than a year later — named BCS after the trio, who shared the Nobel prize (J. Bardeen, L. N. Cooper and J. R. Schrieffer Phys. Rev. 108, 1175; 1957).The work has had far-reaching consequences for both fundamental science and practical technology. Schrieffer continued to make foundational contributions to our understanding of electrons in solids.
Born in Oak Park, Illinois, in 1931, Schrieffer studied physics at the Massachusetts Institute of Technology in Cambridge as an undergraduate. It was at graduate school at the University of Illinois at Urbana-Champaign that he began working with Bardeen, who in 1956 had just won a share of the physics Nobel for the invention of the transistor.
Bardeen suggested Schrieffer try his hand at understanding superconductivity. This was a risky proposition. After the initial success of quantum theory in describing ordinary conductors, insulators and semiconductors, there had been countless attempts to explain superconductors and all had failed. But the timing was right. Bardeen, with his then-postdoc David Pines, had studied the effect of phonons (quantized sound waves) on metals, showing that they mediated an attractive interaction between electrons. Cooper found that this attractive interaction could lead to the formation of bound pairs of electrons. However, Cooper’s theory described only the formation of a single electron pair. The question remained how to describe the many electrons pairing in the full electronic state of the metal, and why such pairing would lead to the properties of a superconductor.
Schrieffer’s intuitive leap came to him on the subway while attending an APS meeting in 1957. It struck him that a natural wave function for describing a state with electron pairing was one in which the number of electrons was not fixed, but had a certain quantum mechanical uncertainty. He wrote it down there and then. This key insight, radical at the time but now part of the standard toolkit of theoretical physics, cracked the problem wide open. With the wave function in hand, it quickly became possible to calculate many of the observed properties of superconductors, and to predict new properties, which were subsequently found.
Schrieffer’s beautiful idea has contributed to many branches of fundamental physics. In condensed-matter physics, it has also been applied to superfluid helium-3 and cold-atom systems. Elsewhere, the theory has helped to explain complex nuclei and neutron stars, and played a crucial part in establishing the understanding of quantum field theory that underlies today’s standard model of strong, electromagnetic and weak interactions.
Schrieffer went on to take postdoctoral positions at the Niels Bohr Institute in Copenhagen and at the University of Birmingham, UK. He held faculty positions at the University of Chicago, the University of Illinois and the University of Pennsylvania.
Throughout his career, Schrieffer displayed the same flair as in his brilliant wave function insight. In 1979, he and his colleagues showed that certain conducting polymers could exhibit excitations with electrical charge, but no spin (the magnetic moment of each electron is called its spin). The opposite could also occur: excitations could have spin, but no charge. It was a revelation that the two fundamental properties of electrons, charge and spin, could be split apart. This deconstruction has since been discovered at many other frontiers of condensed-matter physics. A later collaboration showed that a second example of deconstructed electrons, the fractionally charged excitations in the fractional quantum Hall states, also exhibit fractional statistics, meaning that they are not the conventional bosons or fermions that were thought to divide all fundamental particles into two classes.
In 1980, he moved to the University of California, Santa Barbara, and joined the newly formed Institute for Theoretical Physics. Here, between 1984 and 1989, he served as its second director, helping to establish its strong reputation as a centre for theoretical physics research. His final move in 1992 was back to Florida, where he took a state-wide professorial position in the Florida State University System. From that year until 2006 he was the first chief scientist of the National High Magnetic Field Laboratory at Florida State University in Tallahassee, where he had a crucial role in establishing the new facility’s scientific credentials. His 1996 APS presidency was marked by his efforts to improve communication between the physics community and the public, and between physicists themselves to help unify the field.
Schrieffer was equally known for his warmth, charm, generosity and brilliance. When Bob discussed physics, his eyes would twinkle and a boyish demeanour would shine through. This enthusiasm and provision of wise counsel to younger physicists never waned. His unique style is captured, as if in a photograph, by the BCS wave function.
Read more +
Claude Elwood Shannon was born on April 30, 1916 in Petoskey, Michigan. After attending primary and secondary school in his neighboring hometown of Gaylord, he earned bachelors degrees in both electrical engineering and mathematics from the University of Michigan. After graduation, Shannon moved to the Massachusetts Institute of Technology (MIT) to pursue his graduate studies. While at M.I.T., he worked with Dr. Vannevar Bush on one of the early calculating machines, the "differential analyzer," which used a precisely honed system of shafts, gears, wheels and disks to solve equations in calculus. Though analog computers like this turned out to be little more than footnotes in the history of the computer, Dr. Shannon quickly made his mark with digital electronics, a considerably more influential idea. In a prize-winning masters thesis completed in the Department of Mathematics, Shannon proposed a method for applying a mathematical form of logic called Boolean algebra to the design of relay switching circuits. This innovation, credited as the advance that transformed circuit design “from an art to a science,” remains the basis for circuit and chip design to this day. Shannon received both a master's degree in electrical engineering and his Ph.D. in mathematics from M.I.T. in 1940.
In 1941, Shannon took a position at Bell Labs, where he had spent several prior summers. His war-time work on secret communication systems was used to build the system over which Roosevelt and Churchill communicated during the war. When his results were finally de-classified and published in 1949, they revolutionized the field of cryptography. Understanding, before almost anyone, the power that springs from encoding information in a simple language of 1's and 0's, Dr. Shannon as a young scientist at Bell Laboratories wrote two papers that remain monuments in the fields of computer science and information theory. "Shannon was the person who saw that the binary digit was the fundamental element in all of communication," said Dr. Robert G. Gallager, a professor of electrical engineering who worked with Dr. Shannon at the Massachusetts Institute of Technology. "That was really his discovery, and from it the whole communications revolution has sprung."
Shannon’s most important paper, ‘A mathematical theory of communication,’ was published in 1948. This fundamental treatise both defined a mathematical notion by which information could be quantified and demonstrated that information could be delivered reliably over imperfect communication channels like phone lines or wireless connections. These groundbreaking innovations provided the tools that ushered in the information age. As noted by Ioan James, Shannon biographer for the Royal Society, “So wide were its repercussions that the theory was described as one of humanity’s proudest and rarest creations, a general scientific theory that could profoundly and rapidly alter humanity’s view of the world.” Shannon went on to develop many other important ideas whose impact expanded well beyond the field of “information theory” spawned by his 1948 paper.
Shannon approached research with a sense of curiosity, humor, and fun. An accomplished unicyclist, he was famous for cycling the halls of Bell Labs at night, juggling as he went. His later work on chess-playing machines and an electronic mouse that could run a maze helped create the field of artificial intelligence, the effort to make machines that think. And his ability to combine abstract thinking with a practical approach — he had a penchant for building machines — inspired a generation of computer scientists. Dr. Marvin Minsky of M.I.T., who as a young theorist worked closely with Dr. Shannon, was struck by his enthusiasm and enterprise. "Whatever came up, he engaged it with joy, and he attacked it with some surprising resource — which might be some new kind of technical concept or a hammer and saw with some scraps of wood," Dr. Minsky said. "For him, the harder a problem might seem, the better the chance to find something new."
While Shannon worked in a field for which no Nobel prize is offered, his work was richly rewarded by honors including the National Medal of Science (1966) and honorary degrees from Yale (1954), Michigan (1961), Princeton (1962), Edin- burgh (1964), Pittsburgh (1964), Northwestern (1970), Oxford (1978), East Anglia (1982), Carnegie-Mellon (1984), Tufts (1987), and the University of Pennsylvania (1991). He was also the first recipient of the Harvey Prize (1972), the Kyoto Prize (1985), and the Shannon Award (1973). The last of these awards, named in his honor, is given by the Information Theory Society of the Institute of Electrical and Electronics Engineers (IEEE) and remains the highest possible honor in the community of researchers dedicated to the field that he invented. His Collected Papers, published in 1993, contains 127 publications on topics ranging from communications to computing, and juggling to “mind-reading” machines.
Read more +
In Broken Genius: The Rise and Fall of William Shockley, Creator of the Electronic Age, Joel Shurkin offers the first biography of this important and troubled physicist. His book is a pageturner, which is rare for a scientific biography, but editing problems at times distract from the book’s engaging story. Relying on a collection of Shockley’s extensive private papers at Stanford University, Shurkin paints a nuanced portrait of the physicist, highlighting his scientific achievements and personal shortcomings. To explain the trajectory of Shockley’s life, Shurkin reveals much of his subject’s childhood and family background. According to Shurkin, Shockley was raised in a family that had a strong paranoid streak, which might explain Shockley’s own mental disorders. His father, a mining engineer, encouraged his son’s scientific interests. Shockley’s childhood was also a lonely one, which left him with a severe lack of social skills.
Shockley was educated in physics at MIT and Caltech and later joined the technical staff of Bell Labs. During World War II, he made his first significant contributions to the area of operations research. Applying statistical techniques to the conduct of warfare, he greatly increased the efficiency of flight crews hunting German submarines in the Atlantic Ocean. His research also played a pivotal role in the firebombing of Japanese cities. According to Shurkin, Shockley’s work in operations research might have been his greatest professional achievement. But the war was also a time of personal strain, which led Shockley to a suicide attempt and increasing alienation from his wife, Jean.
After the war, at the request of management at Bell Labs, Shockley organized a new research group to develop a solid-state switch. Among his top recruits were John Bardeen and Walter Brattain. Shurkin claims that Bardeen and Brattain’s discovery of the point-contact transistor was the turning point in Shockley’s life. Shockley, who had not been involved in Bardeen and Brattain’s day-to-day work, was afraid that he would not get any credit for the invention of the transistor. As a result, he isolated himself in an effort to reassert his intellectual primacy. Competing with his own group, Shockley invented the bipolar transistor, and he also produced a seminal textbook, Electrons and Holes in Semiconductors: With Applications to Transistor Electronics (Van Nostrand, 1950). Although his research landed him the Nobel Prize in Physics, his tactics also alienated his collaborators and convinced Shockley’s superiors that he was not of management caliber. As a result, it became increasingly clear to Shockley that his future at Bell Labs was limited.
In the early 1950s, Shockley experienced a mid-life crisis, along with amplified mental and behavioral problems. He divorced his wife, who was then fighting cancer, and married a psychiatric nurse, Emmy Lanning. He also decided to go into business for himself and established the Shockley Semiconductor Laboratory in California. The business was a dismal failure, partly because of Shockley’s propensity to compete with and offend his own staff. But the startup was also the origin of the semiconductor industry in Silicon Valley, as the remarkable group of scientists Shockley had recruited went on to establish major firms such as Fairchild Semiconductor and Intel Corp. By the mid 1960s, Shockley, humbled by his business failure and by the successes of his staff, joined the electrical-engineering faculty at Stanford.
The most interesting part of Shockley’s biography is Shurkin’s discussion of his racist theories of heredity. Using his Nobel Prize fame and showing great skill in manipulating the press, Shockley became the most vocal and visible proponent of eugenics in the US. From the late 1960s through the 1970s he advocated the ideas that intelligence was hereditary and that blacks as a group were less intelligent than whites. He also claimed that the less intelligent should be prevented from having children. His views, expressed during the civil rights movement, led to considerable public outrage, and he was also attacked and vilified by the scientific establishment. His reputation destroyed, Shockley became increasingly isolated and reclusive until his death in 1989.
Broken Genius has a lot going for it. It offers interesting insights into Shockley’s remarkable rise and fiery demise. Yet the book’s production should have been handled with greater care. The biography is riddled with factual mistakes and misspelled names. The narrative is also disjointed at times and would have benefited from more careful editing. In short, Shurkin’s book is an interesting and enjoyable read, but one could have hoped for a better-crafted biography.
Read more +
In 1884, Thomson became Cavendish Professor of Physics. In 1890, he married Rose Paget, and he had two children with her. One of his students was Ernest Rutherford, who would later succeed him in the post.
Thomson's discovery of the electron began in 1895 with a series of experiments in the Cavendish Laboratory. Influenced by the work of James Clerk Maxwell land the discovery of the X-ray, Thomson deduced that cathode rays (produced by Crookes tube) exhibited a single charge-to-mass ratio e m and must be composed of a single type of negatively charged particle, which he called "corpuscles." G. Johnstone Stoney had proposed the term electron earlier as a fixed quantum of electric charge in electrochemistry, but Thomson realized that it was also a subatomic particle, the first one to be discovered.
After further experiments on how cathode rays penetrate gases, Thomson hypothesized that "we have in the cathode rays matter in a new state this matter being the substance from which all the chemical elements are built up." Thomson might be described as "the man who first split the atom," and to a great extent, he made atom physics a modern science. He was awarded the Nobel Prize in Physics in 1906 and was knighted in 1908. His investigations into the action of electrostatic and magnetic fields in the nature of so-called "anode rays" or "canal rays" would eventually result in the invention of the mass spectrometer (then called a parabola spectrograph) by Francis Aston, a tool that allows the determination of the mass-to-charge ratio of ions and which has since become an ubiquitous research tool in chemistry.
Thomson was a gifted lecturer and teacher. His importance in physics is recognized almost as much for those he inspired as for his own experimental work. Seven Nobel Prizes were awarded to those who worked under him, including his son, Sir George Paget Thomson.
Prior to the outbreak of World War I, Thomson made another groundbreaking discovery: the isotope. He died on August 30, 1940, and was buried in Westminster Abbey, close to Isaac Newton.
Read more +
Thomson was a physicist and engineer who embodied the ‘classical approach’ to physical sciences. His work spanned many areas of the discipline including thermodynamics, electrical theory and practice, navigation and the design and manufacture of precision instruments.
The university holds a number of objects which relate to Kelvin’s early involvement with the transatlantic cables, such as a ‘scalmp’ optical galvanometer, the Kelvin bridge (which he used to demonstrate that early copper cables contained too many impurities for conduction), and a piece of submarine telegraph cable from the 1860-1890 made from copper wire.
In addition, there are several pieces of scientific equipment in the collection relating to Kelvin's investigations into electricity and magnetism. Many of his instruments were made by leading instrument manufacturers but he later went on to set up his own factory with the optical, mathematical and philosophical instrument maker James White, manufacturing instruments to a very high standard. Thomson gave his name to the Kelvin scale which measures temperature to absolute zero.
Kelvin's work on calculating the age of the earth based on the rate of the earth’s cooling led him to suggest the earth was 400 million years old. However, he did not account for the effects of radio-active heating from within the earth, as this had yet to be discovered. Kelvin’s estimates of the age of the earth were not old enough to be compatible with Darwin's theory of evolution. This set him at odds with his contemporaries at Aberdeen such as Sir John Struthers and Henry Alleyne Nicholson.
Special Collections Centre holds a section of letters written by Kelvin, discussing time spent with academic collaborators such as the mathematician D. F. Gregory, as well as comments on university life in which he expresses his opinions on funding, staffing and equipment provision.
Read more +
Wilhelm Weber entered the University of Halle in 1822 where he was taught and strongly influenced by the physicist Johann S C Schweigger and the mathematician Johann Friedrich Pfaff. He wrote his doctoral dissertation under Schweigger's supervision on the theory of reed organ pipes and submitted it to Halle in 1826. After that he taught at Halle from 1827 after completing his habilitation thesis on reed organ pipes as coupled oscillators with acoustic coupling of tongue and air cavity. He published a series of papers on this topic between 1828 and 1830 in Annalen der Physik und Chemie :-
One of the subjects treated was the use of this coupling to maintain constancy of pitch of a pipe under different intensities of blowing, and the possibility that this might provide an improved standard of pitch.
His promotion was rapid for after his appointment as a Privatdozent in 1827 he became an Extraordinary Professor of natural philosophy at Halle in the following year. In fact 1828 was quite a significant year for Weber for in September of that year he and his brother Ernst travelled to Berlin to attend the 7th meeting of the Gesellschaft Deutscher Naturforscher und Arzte. The meeting was organised by Alexander von Humboldt who was very impressed with the talk Weber gave on organ pipes. Equally important was the fact that Carl Friedrich Gauss also attended Weber's lecture and immediately saw the tremendous potential displayed by the young physicist. At this time Gauss was interested in geomagnetism and he realised that Weber would make an outstanding co-worker. He spoke to Weber and asked if he would be interested in taking a position in Göttingen if one were to become available. Indeed after the death of Tobias Mayer Jr, Weber was offered a professorship in physics at Göttingen in April 1831 which he immediately accepted. There followed six years of close friendship and collaboration between Weber and Gauss. He soon gained an excellent reputation as a lecturer, illustrating his lectures with experiments. He felt, however, that students could only learn by doing experiments, not simply by watching them carried out, and he opened the physical laboratory at Göttingen for student use.
In 1832 Weber and Gauss published a joint paper which introduced absolute units of measurement of magnetism for the first time. Before this major advance, measurements were made with a pre-calibrated magnetic instrument and were not properly reproducible. This represented an important step forward in the development of magnetism. Weber made major contributions to this work, particularly by developing sensitive magnetometers and other magnetic instruments. Equally important was Weber's later work extending these ideas on magnetic measurements to electrical measurements which we mention again below. This work led to Maxwell's introduction of some aspects of Weber's distant-action theory into his field theory of electricity and magnetism, see. Another joint venture by Weber and Gauss of fundamental importance was their founding of the Göttingen Magnetische Verein in 1833. In the Gauss-Weber telegraph design is discussed. This telegraph was a battery operated line 3000 metres long connecting the Physical Laboratory and the Astronomical Observatory at Göttingen, allowing simultaneous magnetic observations at the two sites. Gauss and Weber jointly published Atlas Des Erdmagnetismus: Nach Den Elementen Der Theorie Entworfen in 1840 which contains magnetic maps constructed using a network of magnetic observatories which they had organized from 1836 onwards to correlate measurements of terrestrial magnetism around the world.
Not all Weber's work during this time was with Gauss, for he also collaborated with his younger brother Eduard, an anatomist and physiologist, who was interested in the physics of human locomotion, particularly the mechanism of walking. They published the joint work Mechanik der menschlichen Gehwerkzenge in 1836. Weber also published several important papers on acoustics during these years.
Political events had already had a major impact on Weber's life when as a boy his family had to leave Wittenberg. After six highly productive years at Göttingen, events again conspired to alter the direction of his life. To understand these events we need to look briefly at the history of Hanover which had come under British influence after the fall of Napoleon in 1814. George IV imposed a constitution on Hanover in 1819 which meant it was dominated by its nobles. An uprising in 1830, shortly before Weber moved to Göttingen, led to William IV introducing a much more liberal and acceptable constitution in 1833. However, William IV died in June 1837 and Hanover separated from Britain with Victoria becoming Queen of Britain while her uncle Ernest Augustus became King of Hanover. As King he repealed the constitution of 1833, which he considered far too liberal. Two weeks after this act by the King, seven professors from Göttingen sent a protest letter to the King explaining that the oath they had taken as professors bound them to the 1833 constitution. Weber was one of the "Göttingen Seven" who signed the protest and among the others were the brothers Jacob and Wilhelm Grimm, the authors of 'Grimm's Fairy Tales'. As explained in Encyclopaedia Britannica:-
Through their part in this protest directed against despotic authority, [the Göttingen Seven] clearly demonstrated the academic's sense of civil responsibilities, manifesting their own liberal convictions at the same time.
All seven professors were dismissed while three of them were ordered to leave the kingdom of Hanover. Weber, although dismissed, was not forced to leave Göttingen and he continued to work at the Göttingen Magnetische Verein without holdig any university position. Gauss and von Humboldt appealed to the King to reinstate Weber and the King agreed to do so provided Weber make a public retraction of the views expressed in the letter of protest. Weber, however, was a man of strong principles and he was certainly not prepared to make a public statement which went totally against his views so he refused to make the required public retraction. He remained at Göttingen without a position until 1843 but he did take the opportunity to travel between March and August 1838. He first visited Berlin before travelling on to London, where he enjoyed useful conversations many English scientists including John Herschel, and finally to Paris where he met most of the leading French scientists. In 1843 he became professor of physics at Leipzig joining his brothers Ernst and Eduard who were both professors at the University. He was appointed to fill G T Fechner's chair at Leipzig after Fechner had to take retirement due to blindness. At Leipzig, Weber continued the work on Ampère's law of electrical force which he had been undertaking in Göttingen from 1832 onwards. He published Elektrodynamische Massenbestimmungen in 1846:-
Weber's greatest theoretical contributions appeared in the 'Elektrodynamische Massenbestimmungen' Ⓣ, seven long works published from 1846 to 1878, besides a manuscript published posthumously. In the first of these, Weber introduced his dynamometer to test Ampère's law of force between electric current elements, to a degree of precision exceeding Ampère's, and also investigated electromagnetic induction.
Dolbear writes:-
Until Weber's work there had been no such thing as electrical measurements. There had been nothing more than comparisons between magnitudes of the same kind. Weber showed how an electrical quantity could be stated in terms of the unit of time, length, and mass, without any reference to other electrical phenomena, and this was a new and great achievement. The British Association Committee on Electrical Standards adopted Weber's work as a basis for their standards of units. Secondly, he was one of the first to feel the necessity for an adequate mechanical conception of electro-magnetic phenomena, and he worked out in a mathematical way, and gave consistency to the idea of molecular magnets, that is, that every molecule of iron is a magnet by constitution, and the various phenomena of the magnetic field are due to the relative positions of these molecules.
D'Agostino writes:-
Gauss and Weber's systematic assignment of absolute units, overshadowed by our four-unit systems, allowed the nineteenth-century analytic formulation of physical laws (a fundamental requisite for theoretical predictions) ...
In fact what Weber achieved was a bringing together of three laws, namely that describing the interactions of two electric charges at rest, Ampère's law for moving electric currents, and the law describing electrical induction.
In 1848 a series of republican revolts against European monarchies spread through France, Germany, Italy, and the Austrian Empire. Ernest Augustus's reign was already a stormy one with trouble between the King and his people and the 1848 revolt forced him to grant Hanover a new much more liberal constitution. The door was thus opened for Weber to return to Göttingen but his position had already been filled by Johann Benedict Listing in 1839 despite the fact that he had never published a paper. Weber insisted that Listing should keep the chair so he returned to Göttingen in 1849 as the Director of the Astronomical Observatory. By this time Gauss was over seventy years of age and rather too old for the two scientists to restart the remarkably fruitful collaboration which had begun nearly twenty years earlier. Gauss died in 1855, and shortly before this Weber began a collaboration with Rudolph Hermann Arndt Kohlrausch who was then at Marburg. Their work on the ratio between the electrodynamic and electrostatic units of charge, published in 1856, proved extremely important and was crucial to Maxwell in his electromagnetic theory of light. Weber found the ratio was 3.1074×108m/sec but failed to take any notice of the fact that this was close to the speed of light. In fact the first use of "c" for the speed of light appears in this paper. Bernhard Riemann, who spent eighteen months as Weber's assistant, was present when the experiments were carried out and he did make the connection between light and both electrodynamic and electromagnetic phenomena.
Weber's later years at Göttingen were devoted to work in electrodynamics and the electrical structure of matter. He was described by Thomas Hirst, who visited Göttingen in the 1850s, in the following way:-
He speaks and stutters on unceasingly, one has nothing to do but listen. Sometimes he laughs for no earthly reason, and one feels sorry at being not able to join him.
Woodruff describes Weber as:-
... friendly, modest and unsophisticated.
Weber never married but his sister often helped manage his household and, in later years, his niece carried out this task. His pleasures outside of his academic work included hiking and he loved to walk for long distances. He died peacefully in the garden of his home in Göttingen having outlived both his scientist brothers, the elder by 13 years and the younger by 20 years. He is buried in the same cemetery as two famous physicists Max Planck and Max Born.
For his outstanding achievements Weber received many honours. He was elected to the Royal Society of London in 1850 and awarded their Copley Medal in 1859. He was elected an honorary fellow of the Royal Society of Edinburgh on 2 March 1874. He was also elected to the American Academy of Arts and Sciences. In 1879 he was awarded the Matteucci Medal by the Italian Society of Sciences. In 1935 the unit of magnetic flux was named the weber in his honour. Actually this is not quite as straightforward as it might at first appear. The unit of electric current is known as the ampere, the term becoming accepted after being proposed by Helmholtz in 1881. In fact around that time the term weber was quite widely used for the unit of electric current but Helmholtz had been in a number of disputes with Weber so he was keen to not have such an important unit named after someone with whom he frequently disagreed. In particular Helmholtz and Weber held different ideas about the nature of the mass of electric corpuscles; see for example [9]. In fact A E Woodruff, in a review of, writes:-
It is the concept of electrical atomicity, as developed by Weber and elaborated by his followers, which represents his chief conceptual contribution to the growth of physics.
Writing in, Woodruff expresses these views in the following way:-
Although he was perhaps most widely known during his life for his law of force, which was discarded with the triumph of Maxwell's field theory. Weber left his more lasting impression on physical theory with his atomistic conception of electrical charge and his vision of the role of such charges in determining the electrical, magnetic and thermal properties of matter.